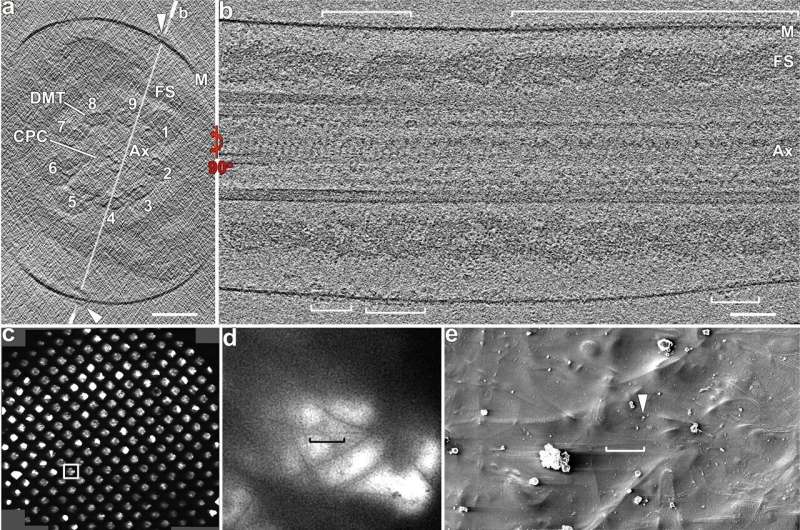
Cryo-ET of whole-cell and cryo-FIB milled mouse sperm flagella reveals rows of CatSper complex particles. a–b Cross-sectional (a) and longitudinal (b) tomographic slices of a representative principal piece region from a whole-cell (i.e., not cryo-FIB milled) wild type mouse sperm flagellum (in non-capacitated state) show CatSper complexes (arrowheads, brackets). Labeled line in (a) indicates the position of the section shown in (b). c–h The cryo-FIB milling workflow consists of: selection of a suitable sample area using an overview (c) and zoom-in (d) cryo-TEM image of an EM grid with plunge-frozen mouse sperm; scanning-EM image recorded in the Dual-Beam/FIB instrument (e) showing the same grid area as in (d); SEM images then show the targeted sperm flagellum before (f) and after (g, h) cryo-FIB milling from top (e, h) and side (f, g) views. For reference, the same particle (black arrowheads) is indicated in all SEM images. i–k A cryo-TEM image (i) of the same cryo-FIB lamella shown in (h) allows selecting an area with a mouse sperm flagellum (boxed area) for tilt series recording. Cross-sectional (j) and longitudinal (k) tomographic slices show better contrast and resolution for the cryo-FIB milled flagellum and CatSper complexes (arrowheads) than for whole-cell samples (compare j, k to a, b). Labeled line in (j) indicates the position of the section shown in (k). Other labels: Ax axoneme, CPC central pair complex, DMT & 1-9, doublet microtubules, FS fibrous sheath, M membrane. Axonemal structures are shown in cross-sectional views from proximal to distal orientation, and in longitudinal views with proximal side of the flagellum on the left in all figures unless otherwise noted. Scale bars: 100 nm in (a, b, j, k); 400 μm in (c); 10 μm in (d, e); 4 μm in (f–h); 1 μm in (i). Credit: Nature Communications (2022). DOI: 10.1038/s41467-022-31050-8
Channels that line the tail of sperm contain pores that allow the entry of calcium, which fuels their journey to the female reproductive tract.
In a new study, researchers from Yale and the University of Texas Southwestern captured images of critical components of these so-called CatSper ion channels, revealing that they are interconnected and arranged in zig-zag fashion. This molecular architecture allows for the highly coordinated whip-like action of the sperm tail that propels it to its goal, they report in the journal Nature Communications.
"The subunits of these channels are arranged like staggered beads on a necklace in a double row," said Jean-Ju Chung, associate professor of cellular and molecular physiology at Yale and co-corresponding author of the research.
Chung and her Yale colleagues had previously identified the CatSper channel complex, which is linearly arranged along the flagella of sperm, as the primary driver of sperm's hyperactivated motility. The use of cryo-electron tomography allowed researchers in the new study to view the channel complex at the atomic level, revealing an important glimpse of the complex relationship of components along the channels which are essential to jumpstarting life in mammals.
Credit: Yale University
More information: Yanhe Zhao et al, 3D structure and in situ arrangements of CatSper channel in the sperm flagellum, Nature Communications (2022). DOI: 10.1038/s41467-022-31050-8
Journal information: Nature Communications
Provided by Yale University