Research team untangles more secrets of intrinsically disordered regions of proteins
Intrinsically disordered regions (IDRs) of proteins, when tethered to folded domains, function either as flexible tails or as linkers between domains. Most IDRs are composed of a mixture of oppositely charged residues. Recent measurements of tethered polyampholytes have shown that arginine- and lysine-rich sequences tend to behave very differently from one another.
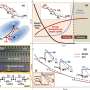
In a paper published May 5 in the Proceedings of the National Academy of Sciences, Rohit Pappu, the Gene K. Beare Distinguished Professor in the Department of Biomedical Engineering at the McKelvey School of Engineering at Washington University in St. Louis, presented research based on computer simulations that showed the differences are determined by differences in free energies of hydration, steric volumes and other considerations.
Further, the interplay between electrostatic attractions and favorable free energies of hydration creates distinct stable states for polyampholytic IDRs. These findings have implications for switch-like transitions and the regulation of effective concentrations of interaction motifs by IDRs.
More information: Xiangze Zeng et al, Competing interactions give rise to two-state behavior and switch-like transitions in charge-rich intrinsically disordered proteins, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2200559119
Journal information: Proceedings of the National Academy of Sciences
Provided by Washington University in St. Louis