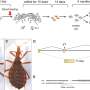
Graphical abstract. Credit: Structure (2022). DOI: 10.1016/j.str.2022.04.004
An international team of researchers have discovered how a tumor-promoting kinase evolves into a GTP sensor kinase.
Led by researchers from the University of Cincinnati and Japan's the University of Tokyo, High Energy Accelerator Research Organization (KEK), Keio University, Rikkyo University, Hoshi University, Tokai University and Kansai Medical University, the findings were published May 2 in the journal Structure.
Atsuo Sasaki, Ph.D., one of the research team's lead investigators, said the findings provide a critical insight that connects the dots among the ATP preference of kinases, GTP-recognition of G-proteins and evolutionary mechanism of nucleotide specificity and could lead to the development of a new cancer-treating drug that targets the GTP-sensor kinase PI5P4Kβ using the discovered structural information that renders it to utilize GTP instead of ATP.
"Principally, kinases use ATP to phosphorylate and control their substrates, and many diseases, including cancers, are caused by the dysregulation of kinases. Being able to modulate the kinase activity is central to fight against cancers and multiple diseases," said Sasaki, associate professor at the University of Cincinnati College of Medicine.
Kinases are a type of enzyme essential for various cellular processes, including signal transduction, transcription and metabolism. Protein kinases, which represent the largest superfamily consisting of more than 500 genes in the human genome, phosphoinositide kinases (PI-kinases) and inositol phosphate kinases (IP-kinases, including inositol kinases) share structural motifs that serve for ATP recognition, followed by hydrolysis and phosphotransfer to their substrates.
Although there is extraordinary diversity in their structure, substrate specificity and participating pathways, all these kinases use ATP as the physiological phosphate donor. However, how and why kinases possess the ATP preference among other nucleoside triphosphates remains largely unknown, Sasaki said.
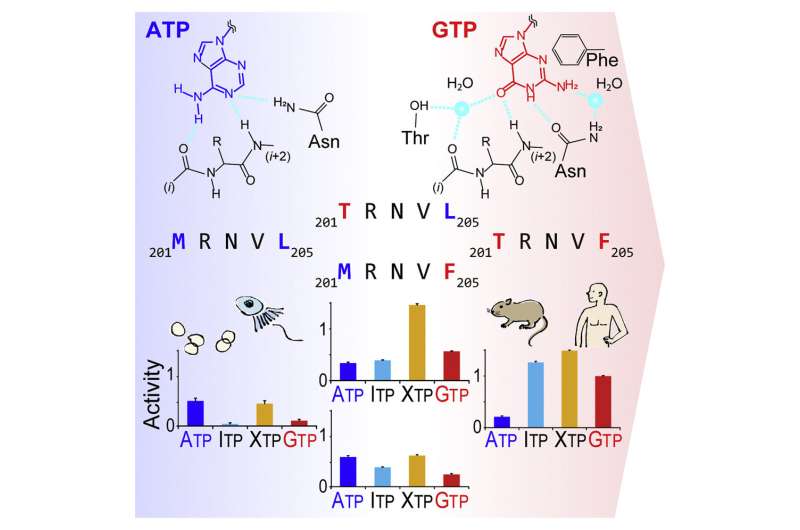
"Thus, we were surprised when we found the strong GTP-preference of phosphatidylinositol 5-phosphate 4-kinase β (PI5P4Kβ)," said Sasaki. "Our previous studies show that PI5P4Kβ acts as an intracellular GTP-sensor and regulates tumorigenesis and stress responses. But, we did not know how this peculiar GTP-reactivity is acquired, from which my team set out to explore.
"To understand the mechanism of GTP-dependence, it is critical to know how kinases recognize ATP. We were surprised that there had been no comprehensive study about the ATP recognition mechanism by kinases," Sasaki adds.
Sasaki said there are more than 600 kinases, including protein and phosphoinositide kinases, whose structures are solved in the ATP-bound form. However, these data are deposited individually. Regardless of extensive efforts to target kinases for cancer therapy, the precise mechanism of how kinases recognize ATP was unclear.
The multidisciplinary, international team was organized and firstly conducted a structural comparison of 661 kinases and 128 G-proteins that utilize GTP, unveiling a common mechanism for ATP recognition by kinases. Then, the research team investigated the GTP-recognizing mechanism of PI5P4Kβ by a combination of biochemical and structural analyses using a nuclear magnetic resonance (NMR) activity assay followed by an X-ray structural study.
In addition, a cutting-edge technology called the fragment molecular orbital (FMO) calculation enabled the team to identify the critical amino acid residues and the protein-nucleotide interactions that make the PI5P4Kβ a GTP-reactive kinase.
The evolutionary retrograde mutations that turn back time on the evolution of the GTP-utilizing PI5P4Kβ from the ATP-utilizing kinase PI4P5K unveiled two critical mutations in a short stretch of sequence, which the researchers named the guanine-efficient association (GEA) motif, that endowed PI5P4Kβ the GTP sensing activity.
"Through our multinational cross-disciplinary collaboration and the team's more than five years of hard work, we were able to understand how the dogmatic rule of ATP-utilization by kinase can be overturned to the GTP preference in PI5P4Kβ," Sasaki said. "We are excited to continue our research on PI5P4Kβ to develop therapies to eliminate cancers that increase the dependence of this GTP-sensor kinase."
More information: Koh Takeuchi, The GTP-responsiveness of PI5P4K? is evolved by a compromised trade-off between activity and specificity., Structure (2022). DOI: 10.1016/j.str.2022.04.004. www.cell.com/structure/fulltex … 0969-2126(22)00131-9
Journal information: Structure
Provided by University of Cincinnati