Credit: Malidate Van from Pexels
The amount of salt and water in our cells and their pH is strictly controlled for cell survival. To maintain the necessary balance, special proteins perform the essential role of exchanging protons (hydrogen ions, or H+) for sodium (Na+) or lithium (Li+) ions across cell membranes. These proteins are called sodium-proton exchangers (Na+/H+ exchangers, or NHEs). Found in every cell, NHEs tightly regulate the cells' pH, sodium levels and volume by moving Na+ into the cell in exchange for H+. Scientists have found that when these proteins do not work properly, they may lead to diseases such as cancer, diabetes, heart failure and hypertension.
An NHE of note is NHA2, a protein found in the membrane of kidney cells that control blood pressure and beta cells that control blood glucose levels by producing, storing and releasing insulin. The salt-absorbing NHA2 was recently identified as the long sought-after sodium-proton exchanger linked to hypertension and diabetes in humans. However, despite its importance, scientists knew very little about its structure and how it works.
Researchers supported by the EU-funded EXCHANGE project have determined what NHA2 looks like and how it adapts to the membrane. The new insight gained on this key biological mechanism could lead to the development of new drugs against the two previously mentioned diseases. The scientists' findings are described in a paper published in the journal Nature Structural & Molecular Biology.
To obtain their results, the research team combined electrophysiology, biochemistry, molecular dynamics simulations, native mass spectroscopy and cryogenic electron microscopy. As reported in a news item posted on the website of EXCHANGE project coordinator Stockholm University, Sweden, this led to the discovery of NHA2's structure. It also enabled the researchers to identify how the protein rearranges itself when a specific lipid is present, in order to become more active.
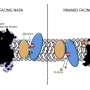
An extra helix
NHA2 consists of 14 transmembrane segments, rather than the 13 segments previously observed in mammalian and related bacterial NHEs. "The additional N-terminal helix in NHA2 forms a unique homodimer interface with a large intracellular gap between the protomers, which closes in the presence of phosphoinositol lipids," the authors write in the study. They then go on to suggest that the extra N-terminal helix serves as a lipid-mediated remodeling switch that regulates NHA2 activity. "These findings reveal a unique adaption of a salt-transporter to a membrane environment with important physiological ramifications," the news item explains.
More information: Rei Matsuoka et al, Structure, mechanism and lipid-mediated remodeling of the mammalian Na+/H+ exchanger NHA2, Nature Structural & Molecular Biology (2022). DOI: 10.1038/s41594-022-00738-2
EXCHANGE project: cordis.europa.eu/project/id/820187
Journal information: Nature Structural & Molecular Biology
Provided by CORDIS