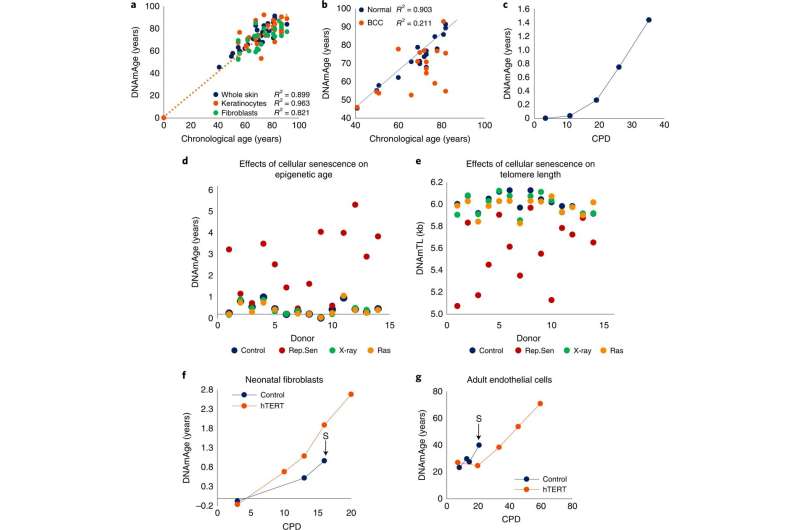
EpiAge is distinct from cellular senescence and telomere attrition. a, Measurement of EpiAge (DNAmAge) of whole skin, keratinocytes and fibroblasts from 14 healthy human skin samples. Absence of samples between 1 and 40 years old reflects the scarcity of hospital admissions of this demographic group. b, Measurement of DNAmAge of 17 BCCs and corresponding adjacent healthy skin. c, EpiAge of in vitro-cultured neonatal human dermal keratinocytes (HDK) derived from foreskin. CPD, cumulative population doubling. Representative of more than three experiments. d, EpiAges of primary human dermal fibroblasts isolated from skin of 14 healthy neonatal donors and subjected to 20 Gy X-rays, transduced to express oncogenic ras or cultured until replicative senescence (Rep.Sen). e, DNA methylation-based estimation of telomere length (DNAmTL) of cells described in d. f, DNAmAge of neonatal primary human dermal fibroblasts transduced with empty vector (control) or hTERT-expressing vector (hTERT). The arrow and letter ‘S’ denote the point at which the untreated control cells became senescent. Representative of two experiments. g, EpiAge of adult HCAECs transduced with empty vector (control) or vector expressing hTERT. Representative of three experiments. Credit: Nature Aging (2022). DOI: 10.1038/s43587-022-00220-0
A team of researchers affiliated with a host of institutions in the U.K. and the U.S. has conducted an investigation into whether epigenetic aging is the manifestation of one or more aging hallmarks. In their paper published in the journal Nature Aging, the group describes subjecting human cells to three kinds of abuse and then testing them to see if the cells aged epigenetically.
Over the past several years, some researchers focusing on the science of aging have become proponents of what is described as epigenetic aging, whereby certain attributes of our bodies age at a rate that may not be consistent with our biological age. That has led to studies aimed at measuring the epigenetic age of people (and other animals) using DNA methylation clocks, ostensibly as a means to circumvent them and allow people to live longer. In this new effort, the researchers studied hallmarks of aging such as exposure to radiation, reproduced them and tested the effects on the pace of epigenetic aging.
The work involved collecting tissue samples from 14 healthy people and dividing them into four groups. One group was subjected to a small dose of radiation, another had some of their cell properties altered to become cancerous, and yet another set was subjected to induced senescence. The fourth group was left undisturbed. Each of the groups represented a hallmark of aging. Exposure to radiation can, for example, make changes to the genome that results in accelerated aging.
None of the tissue samples exhibited changes in epigenetic aging. But the researchers did find changes to the way the cells handled energy—their ability to sense nutrients was impacted. This ability plays a major role in cell growth, reproduction and death. The researchers also found changes in mitochondrial activity and in the number of stem cells in their samples. They suggest that epigenetic aging does not predict changes in senescence, nor does it match with age-related changes to telomeres, one of the major indicators of aging in general.
More information: Sylwia Kabacik et al, The relationship between epigenetic age and the hallmarks of ageing in human cells, Nature Aging (2022). DOI: 10.1038/s43587-022-00220-0
Steve Horvath et al, DNA methylation clocks for dogs and humans, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2120887119
Journal information: Proceedings of the National Academy of Sciences , Nature Aging
© 2022 Science X Network