Discovery uncovers need for ammonia emission regulations

A discovery by former Carnegie Mellon Ph.D. student, Mingyi Wang, leading a large collaborative team, sheds light on one way new particles are forming in the upper troposphere. The study, published in Nature, reveals an unexpected volatile reaction between nitric acid, sulfuric acid, and ammonia, synergistically creating new particles at a rapid rate. The findings suggest that in addition to carbon dioxide, there are other compounds in need of attention and regulation.
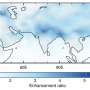
The presence of ammonia was first discovered in the upper troposphere in 2016 using the analysis of averaged MIPAS (Michelson Interferometer for Passive Atmospheric Sounding) infrared limb-emission spectra. Scientists from the Karlsruhe Institute of Technology (Germany), University of Colorado Boulder, and Universidad Nacional Autónoma de México performed a "CT Scan" of the atmosphere, moving along latitudes and longitudes, measuring particle concentrations and compositions in the upper troposphere.
Ammonia is primarily derived from agriculture and vehicles in condensed city settings. When scientists detected the compound in the upper troposphere, they were surprised by how far it had traveled into the atmosphere, raising questions about how it's transported there and its effect on particle mass and creation.
After learning about the former study, Wang, a Ph.D. student in Carnegie Mellon's Department of Chemistry, became interested in the reaction between ammonia, nitric acid, and sulfuric acid in the atmosphere. In a 2020 study, also published in Nature, Wang discovered that in cold conditions, like that of the winter climate in Beijing, the mixture of these three agents contributes and condenses onto nanometer particles, increasing their mass quickly.
With this finding, Wang became curious about what this reaction would look like in even colder, more extreme regions, so he began designing an experiment to test it in upper troposphere-like conditions.
"There are a very limited number of instruments available to identify the processes that create particles in the upper troposphere," said Wang. "We must rely on lab experiments to understand what can occur in those conditions."
To analyze this, Wang headed to Switzerland as a member of the CLOUD collaboration to test his experiment at the European Council for Research Nuclear (CERN). Using their chamber facility, Wang created precisely controlled atmospheric conditions and observed reactions in real-time. When it came time to add ammonia to the chamber, Wang expected to see the mixture of acids and base condense onto existing particles and increase their mass, as he had previously discovered. However, to his surprise, he saw a burst of new particles forming rapidly.
"What we found is that the nitric acid and ammonia are susceptible to temperature changes. When the temperature gets colder, they can actually go through the gas to particle conversion process, creating new particles and increasing the overall particle number concentration," said Wang.
"This is important, especially in the relatively clean upper troposphere. The emission sources are limited up there. There are no factories or farms, and planes are thought to make up most of the pollutants in this area. Any pollutants in the upper troposphere will play a very different role than they do in the boundary layer (the lowest part of the troposphere, directly impacted by the presence of the earth's surface). The temperature and the interplay between species in each are also very different."
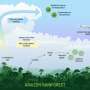
Collaborating with a number of world-renowned climate scientists, including Carnegie Mellon Engineering Assistant Research Professor Hamish Gordon, Wang and his fellow researchers conducted global modeling simulations, demonstrating how ammonia is transported to the upper troposphere and later dispersed.
Additionally, CMU Chemical Engineering Ph.D. student and co-author Brandon Lopez found that even a tiny amount of sulfuric acid could turn particles into formidable ice nuclei.
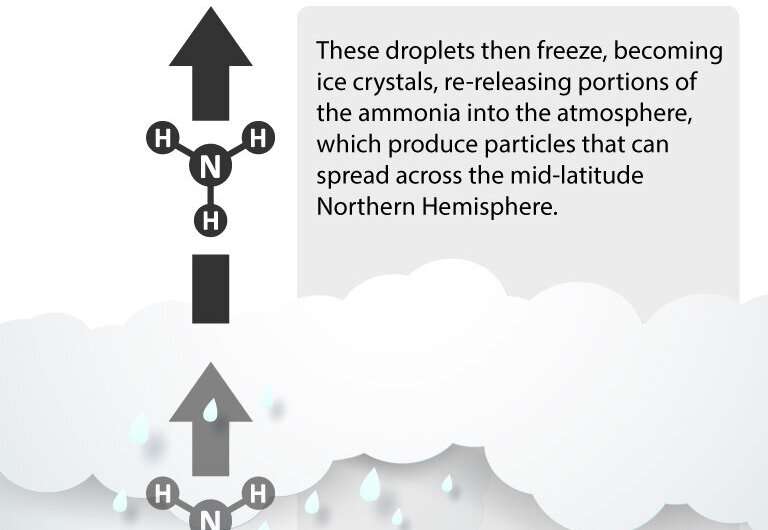
The group uncovered that the ammonia is convected upward during events like the Asian monsoon. Being that ammonia is very soluble, as it passes through clouds, it begins dissolving into cloud droplets. These droplets then freeze, becoming ice crystals, re-releasing portions of ammonia into the atmosphere, producing particles that can spread across the mid-latitude Northern Hemisphere.
"This finding leads us to question whether or not other species, such as organic compounds, can also be transported to the upper troposphere through this process," said Wang.
Wang's advisor, co-author, and world-class climate scientist, Neil Donahue, explained the importance of understanding the possible array of compounds that could be convected and their potential impact.
"All of the scientific uncertainty around climate change relates to clouds in one way or another," said Donahue, a Thomas Lord Professor in the Chemistry, Chemical Engineering, and Engineering and Public Policydepartments at Carnegie Mellon. "To make clouds, you need water to nucleate or freeze."
"In polluted parts of the atmosphere closer to the ground, such as that over big cities, the agents and particles that act as cloud nuclei (seeds) are abundant, but they are quite rare in the vast areas of the upper atmosphere. The nature of clouds can change a lot depending on the type and amount of particles present, so having these particles making and changing cloud composition in the upper atmosphere could significantly impact climate."
Reducing carbon dioxide (CO2) emissions continues to be a major focus of climate scientists and lawmakers. While Wang says reducing CO2 by decreasing fossil fuel combustion will help lower several other pollutants, he believes it's imperative to start developing regulations focused specifically on ammonia emissions.
"We know we have to reduce sulfur and nitrogen oxide emissions from coal power plants and vehicles, but now it's evident we should be thinking about reducing ammonia emissions from vehicles and agriculture too. It's proving to play a critical role both in the boundary layer, affecting air quality, but also in the composition of the upper troposphere."
Wang, now a postdoc at the California Institute of Technology, says the next step is to design additional studies to uncover whether other compounds are making it to the upper troposphere in a similar fashion.
More information: Mingyi Wang et al, Synergistic HNO3–H2SO4–NH3 upper tropospheric particle formation, Nature (2022). DOI: 10.1038/s41586-022-04605-4
Journal information: Nature