How one of the X chromosomes in female embryonic stem cells is silenced
In most mammals, females have two X chromosomes and males have one X and one Y chromosome in each of their cells. To avoid a double dose of X-linked genes in females, one of the Xs is silenced early in the developmental process. This silencing is critical, yet how it happens has been relatively mysterious. Two new U-M studies reveal more about this silencing process and insights that could improve stem cell research.
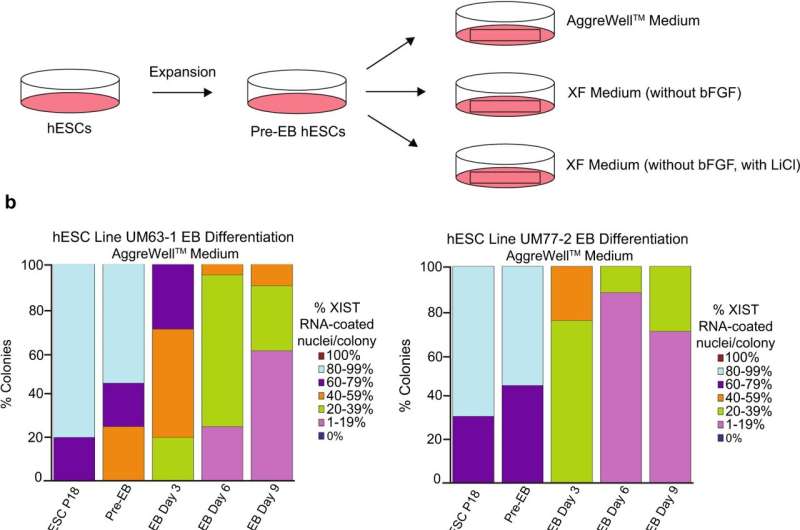
Human embryonic stem cells (hESCs) hold enormous promise for research into early development as well as for regenerative medicine for diseases ranging from type 1 diabetes to Parkinson's disease. Yet, biologists working with female hESCs in the lab often run into a phenomenon wherein the normally inactivated X chromosome loses this suppression while growing in a culture dish.
"If you can't maintain hESCs exactly as such in culture then you can't use them for any downstream application," said Sundeep Kalantry, Ph.D., associate professor of human genetics. He, along with Marissa Cloutier, a Ph.D. trainee, and their team set out to determine why X-inactivation erodes under certain experimental conditions over time.
Their primary suspect was the substance used to grow the cells in culture, called media. Cells are grown in media that supply them with chemical instructions called growth factors. These growth factors signal stem cells to keep dividing. One popular medium, called mTeSR1, appeared to be correlated with the loss of a key regulator of X-inactivation, a non-coding strand of RNA called XIST. Another medium, called Xenofree, did not lead to a loss of X-inactivation.
"We looked at the differences in the composition of these two media and identified lithium chloride as being present in mTeSR1 but not in Xenofree," said Cloutier.
Lithium chloride is sometimes included in media to promote stem cell proliferation, however, it is known to interfere with many cell-signaling pathways by inhibiting GSK-3 proteins. (Inhibitors of GSK-3 proteins have been used to treat several diseases, and lithium, used to treat bipolar disorder, was one of the first natural GSK-3 inhibitors discovered.)
To confirm lithium chloride as the culprit, they added the compound to the Xenofree medium and saw a loss of X-inactivation. Their paper is published in Nature Communications.
"Broadly in terms of our understanding of X inactivation, our study provides a possible new model for the regulation of this process," says Cloutier. Kalantry adds that their study suggests that researchers need to be a little more cautious about the use of GSK-3 inhibitors like lithium. "They may not only interfere with X inactivation, but other modes of epigenetic transcriptional regulation across the genome."
Role for an ancient X-linked gene in inducing X-inactivation
A separate paper, also published in Nature Communications by Kalantry and colleagues, yields insights into the evolution of X-inactivation in mammals. The premise of the study was that X-inactivation is triggered by one or more of a subset of X-linked genes that paradoxically escape X-inactivation once X-inactivation has commenced. These genes are expressed from both X chromosomes in females vs. a single X in males, and as a result are more highly expressed in females vs. males. Kalantry's lab in close collaboration with Shigeki Iwase's lab, also at the University of Michigan, found that due to the higher expression in females vs. males of one such X-linked gene, Kdm5c, it induces Xist and thus X-inactivation selectively in females.
Deleting Kdm5c on both X chromosomes in a female cell turned off X-inactivation almost completely. Conversely, when the researchers introduced Kdm5c into male mouse cells, they successfully initiated X-inactivation, which under normal circumstances would not occur.
Kdm5c is an ancestral X-linked gene that is shared by all mammalian species. The authors tested and found that Kdm5c from evolutionarily distant marsupial and monotreme (e.g., the egg-laying platypus) mammalian lineages, which split from placental mammals more than 150 million years ago, could also remarkably induce Xist and X-inactivation in male mouse cells. This finding suggests that Kdm5c has retained an ancestral function to induce Xist and X-inactivation in mice and humans.
"If expressing X-linked genes at higher-than-normal-levels in a female cell, that cell will often suffer and die. In males, if you reduce expression of X-linked genes beyond normal levels by inactivating that single X, the cells also suffer and die," said Kalantry. "The proper level of X chromosome genes is really, really important to the health of the cell."
The team hopes next to explain the random process that determines which X in a female cell is silenced: the one from the mother or the one from the father. Determining this basic mechanism, says Kalantry, could in the future be applied to reactivate X-linked genes at will for potential therapeutic purposes.
More information: Marissa Cloutier et al, Preventing erosion of X-chromosome inactivation in human embryonic stem cells, Nature Communications (2022). DOI: 10.1038/s41467-022-30259-x
Milan Kumar Samanta et al, Activation of Xist by an evolutionarily conserved function of KDM5C demethylase, Nature Communications (2022). DOI: 10.1038/s41467-022-30352-1
Journal information: Nature Communications
Provided by University of Michigan