Researchers demonstrate, for the first time, an epoxide ring-opening reaction catalyzed by zirconocene under visible light. Credit: Waseda University
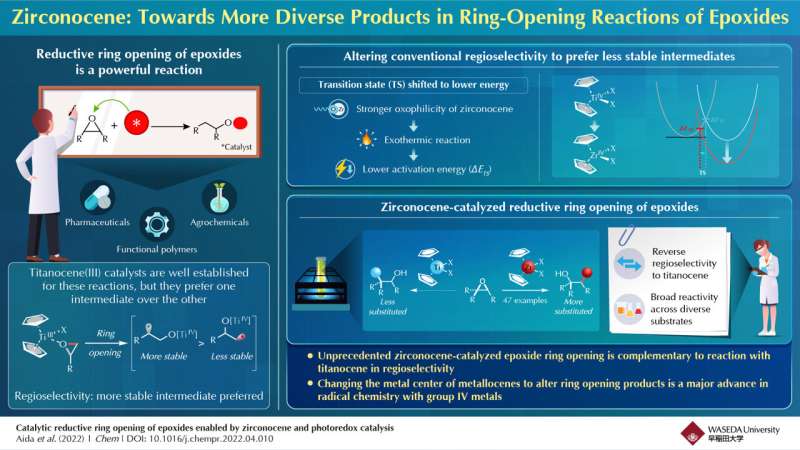
Epoxides belong to a class of organic compounds called "cyclic ethers" that are characterized by a three-atom ring. They are readily available compounds found in medicinal and agrochemical agents, as well as natural products. Epoxides are a valuable industrial precursor as they allow the synthesis of a diverse range of important alcohols, functional polymers, agrochemicals, and pharmaceuticals through a reductive ring-opening reaction. For the last 30 years, titanocene(III) has been the representative, unique catalyst for catalyzing the ring-opening reaction. However, titanocene-catalyzed reactions are regioselective, meaning some products are preferred over others. In its case, the preferred products are those obtained from more stable radicals (as opposed to less stable radicals). The mechanism underlying this regioselectivity is still unclear.
In a new study published in Chem, a team of chemists from Japan led by Professor Junichiro Yamaguchi, graduate students Kazuhiro Aida and Marina Hirao, and Assistant Professor Eisuke Ota from the Department of Applied Chemistry at Waseda University, investigated zirconocene, the zirconium counterpart to titanocene, as a potential alternative catalyst for the ring opening reaction. "Zirconium is more oxophilic compared to titanium, which means it has a higher tendency to interact with oxygen atoms. This changes the pathway of the chemical reaction. We found that changing the titanium metal center to zirconium makes the ring-opening reaction more exothermic, which decreases the activation energy required for ring opening," explains Ota. In their work, the team reported, for the first time, a zirconocene-based catalysis of epoxide ring opening in presence of visible light alone.
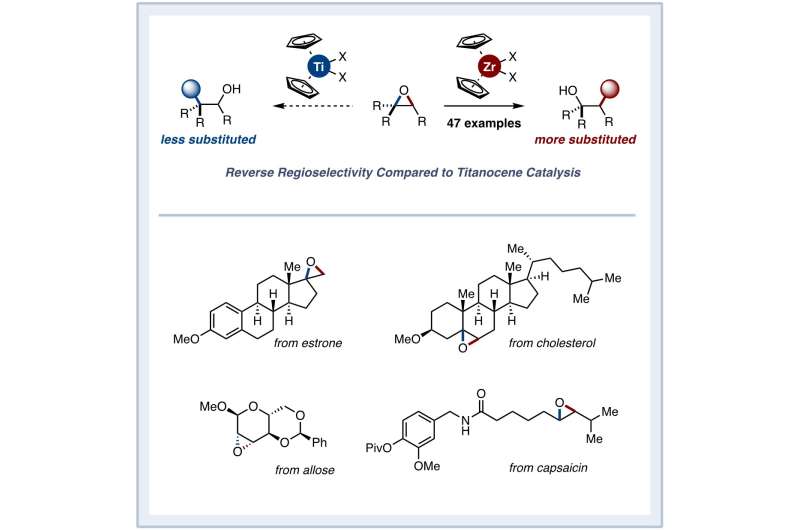
Encouraged by their findings, the researchers demonstrated their novel approach for a broad range of substrates and functional groups, including natural products. The most interesting aspect of their approach, however, was that zirconocene-catalyzed reactions led to products with opposite, complementary regioselectivity compared to those obtained with titanocene-catalyzed reactions. This enabled a readily accessible route to many elusive alcohol products that could not be obtained before owing to regioselectivity issues.
A team of chemists from Waseda University, Japan, recently demonstrated a zirconocene-based photoredox catalysis reaction that enables a reductive ring opening of epoxides with a reversed regioselectivity compared to that for titanocene-mediated reactions. Credit: Junichiro Yamaguchi from Waseda University
Moreover, zirconium offers yet another advantage over titanium. As Yamaguchi explains, "Zirconium is one of the most abundant elements in Earth's crust, which makes it readily available and low-cost. Since our reaction can be catalyzed using visible light, it is also environmentally friendly. With further improvement, our method could be a key contributor to green chemistry."
Overall, the results of this study bring in a new perspective that could go a long way in significantly broadening the scope of reductive epoxide ring-opening reactions and adding a new dimension to radical chemistry.
More information: Kazuhiro Aida et al, Catalytic reductive ring opening of epoxides enabled by zirconocene and photoredox catalysis, Chem (2022). DOI: 10.1016/j.chempr.2022.04.010
Journal information: Chem
Provided by Waseda University