Structure of methane. Credit: Christinelmiller/Wikimedia Commons, CC BY-SA 4.0
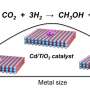
Selective oxidation of methane (CH4) to value-added chemicals with both high catalytic activity and selectivity under mild conditions remains challenging. Due to the low activity of oxygen and the overoxidation of the oxygenates, selective oxidation of CH4 to oxygenates with O2 or O2/H2 suffers from low catalytic activity and low oxygenates selectivity. Moreover, the high loading of noble metals for supported catalysts leads to high cost.
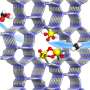
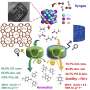
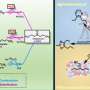
Recently, a research team led by Prof. Sun Yuhan and Prof. Zhong Liangshu from the Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences reported a ZSM-5 (Z-5)-supported PdCu catalyst for selective oxidation of CH4 to oxygenates using O2 in the presence of H2. The catalyst exhibited a high oxygenates yield of 1178 mmol/gPd/h with oxygenates selectivity of 95% at 120 °C.
The results were published in Angewandte Chemie International Edition.
Based on a combination of control experiments and electron paramagnetic resonance as well as in situ spectroscopic techniques, the researchers found that PdO nanoparticles facilitated in situ generation of H2O2. Cu single atoms not only accelerated the generation of abundant ·OH from H2O2 decomposition, but also enabled the homolytic cleavage of CH4 by ·OH to ·CH3. Subsequently, the ·OH reacted quickly with the ·CH3 to form CH3OH with high selectivity.
These findings may provide valuable insights into selective oxidation of methane to oxygenates and shed light on other highly efficient and low-price catalysts for the selective activation of C-H bonds in light alkanes.
More information: Bo Wu et al, Tandem Catalysis for Selective Oxidation of Methane to Oxygenates Using Oxygen over PdCu/Zeolite, Angewandte Chemie International Edition (2022). DOI: 10.1002/anie.202204116
Journal information: Angewandte Chemie International Edition
Provided by Chinese Academy of Sciences