Graphical abstract. Credit: ACS Sustainable Chemistry & Engineering (2022). DOI: 10.1021/acssuschemeng.2c00800
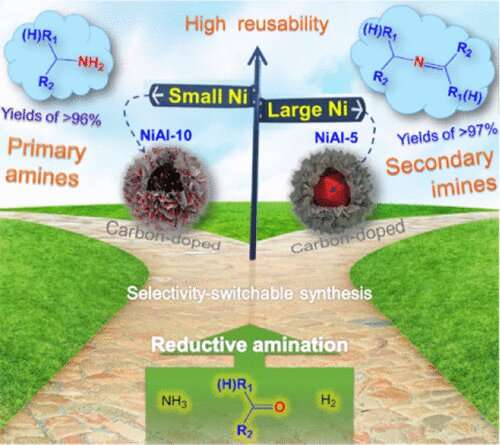
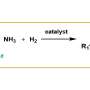
Primary amines and secondary imines are key intermediates widely used in fields like food additives, pharmaceuticals, and agrochemicals. They can be produced via the reductive amination of carbonyl compounds.
However, during the reductive amination process, many side reactions often occur, resulting in poor selectivity toward the target products and correspondingly increasing the cost of purification.
Recently, a research team led by Prof. Wang Guanghui from the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) of the Chinese Academy of Sciences (CAS) has reported a facile strategy to synthesize two carbon-doped Ni catalysts with particle sizes of 7.5 and 47.5 nm, respectively.
This work was published on March 11 in ACS Sustainable Chemistry & Engineering.
The selectivity in the reductive amination of biomass-derived carbonyl compounds is completely switched between primary amines and secondary imines.
"The synthesis is derived from the confined pyrolysis of Ni-Al layered double hydroxides grafted on hollow polymer nanospheres, in which the hollow polymer nanospheres play four roles, i.e., template, reductant, carbon resource, and Ni size controller. The two catalysts exhibit excellent stability due to the existence of surface Ni-Cx species," said Pan Zhengyi, first author of the study.
"Reductive aminations over these two catalysts have a broad substrate scope and can be scaled up to the gram level, showing the potential for industrial applications," Prof. Wang said.
More information: Zhengyi Pan et al, Size-Tunable Carbon-Doped Ni Nanoparticles for Switchable Reductive Amination of Biomass-Derived Carbonyl Compounds to Primary Amines and Secondary Imines, ACS Sustainable Chemistry & Engineering (2022). DOI: 10.1021/acssuschemeng.2c00800
Provided by Chinese Academy of Sciences