Using a permanently unlocked form of the plant enzyme MAX3, UC Davis researchers are exploring the mechanism by which the plant hormone strigoractone regulates a massive network of genes controlling plant growth and architecture. Credit: Nitzan Shabek/UC Davis
As sessile organisms, plants have to continually adapt their growth and architecture to the ever-changing environment. To do so, plants have evolved distinct molecular mechanisms to sense and respond to the environment and integrate the signals from outside with endogenous developmental programs.
New research from Nitzan Shabek's laboratory at the UC Davis College of Biological Sciences, published in Nature Plants, unravels the underlying mechanism of protein targeting and destruction in a specific plant hormone signaling pathway.
"Our lab aims at deciphering sensing mechanisms in plants and understanding how specific enzyme function can be regulated at the molecular levels," said Shabek, assistant professor of biochemistry and structural biology in the Department of Plant Biology. "We have been studying a new plant hormone signal, strigolactone, that governs numerous processes of growth and development, including branching and root architecture."
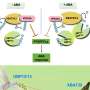
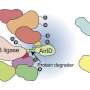
The work stems from a study by Shabek, published in Nature in 2018, unraveling molecular and structural changes in an enzyme, MAX2 (or D3) ubiquitin ligase. MAX2 was found in locked or unlocked forms that can recruit a strigolactone sensor, D14, and target for destruction a DNA transcriptional repressor complex, D53. Ubiquitins are small proteins, found in all eukaryotes, that "tag" other proteins for destruction within a cell.
To find the key to unlock MAX2 and to better understand its molecular dynamics in plants, postdoctoral fellows Lior Tal and Malathy Palayam, with junior specialist Aleczander Young, used an approach that integrated advanced structural biology, biochemistry, and plant genetics.
"We leveraged structure-guided approaches to systemically mutate MAX2 enzyme in Arabidopsis and created a MAX2 stuck in an unlocked form," said Shabek. "Some of these mutations were made by guiding CRISPR/Cas9 genome editing, thus providing us a discovery platform to study and analyze the different signaling outputs and illuminate the role of MAX2 dynamics."
Regulating a massive gene network
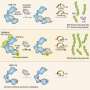
They found that in the unlocked conformation, MAX2 can target the repressor proteins and biochemically decorate them with small ubiquitin proteins, tagging them for destruction. Removing these repressors allows other genes to be expressed—activating a massive gene network that governs shoot branching, root architecture, leaf senescence, and symbiosis with fungi, Shabek said.
Sending these repressors to the proteasome disposal complexes requires the enzyme to relock again. The team also showed that MAX2 not only targets the repressor proteins, but once it is locked, the strigolactone sensor itself is destroyed, returning the system to its original state.
Finally, the study uncovered the key to the lock, an organic acid metabolite that can directly trigger the conformational switch.
"Beyond the implication in plant signaling, this is the first work that placed a primary metabolite as a direct new regulator of this type of ubiquitin ligase enzymes and will open new avenues of study in this direction," Shabek said.
Additional coauthors on the paper are specialist Mily Ron and Professor Anne Britt, Department of Plant Biology. X-ray crystallography data was obtained at the Advanced Light Source, Lawrence Berkeley National Laboratory, a U.S. Department of Energy user facility.
More information: Lior Tal et al, A conformational switch in the SCF-D3/MAX2 ubiquitin ligase facilitates strigolactone signalling, Nature Plants (2022). DOI: 10.1038/s41477-022-01145-7
Journal information: Nature Plants , Nature
Provided by UC Davis