Cell biology: How mitochondria report stress
Ludwig Maximilian University of Munich researchers have discovered the mechanism by which the protein DELE1 detects organelle stress. This offers a possible new approach for treating neurodegenerative diseases.
Researchers have long posited a link between dysfunctions in mitochondria, little organelles in the interior of cells, and the aging process and age-related illnesses, such as Alzheimer's disease. "Many such illnesses cannot be cured—partly because we don't yet understand fundamental mechanisms," says Professor Lucas Jae from LMU's Gene Center Munich.
Frequently, the mitochondrial dysfunction is triggered by various forms of stress—this much is known. Stress can come from the cell or originate in the mitochondrion itself, such as through reactive oxygen species, which occur during cellular respiration. Although they have their own genome, mitochondria are incapable of responding independently to stress. "This means that disturbances must be reported to the rest of the cell," explains Dr. Evelyn Fessler from the Gene Center Munich.
In Nature Communications, Fessler and Jae, together with Luisa Krumwiede, describe a mechanism whereby a special protein in humans, DELE1, detects various kinds of stress while being imported into mitochondria and reports them to the cell. This can lead to different responses, such as repairs or induced cell death.
Known molecule, unknown mechanism
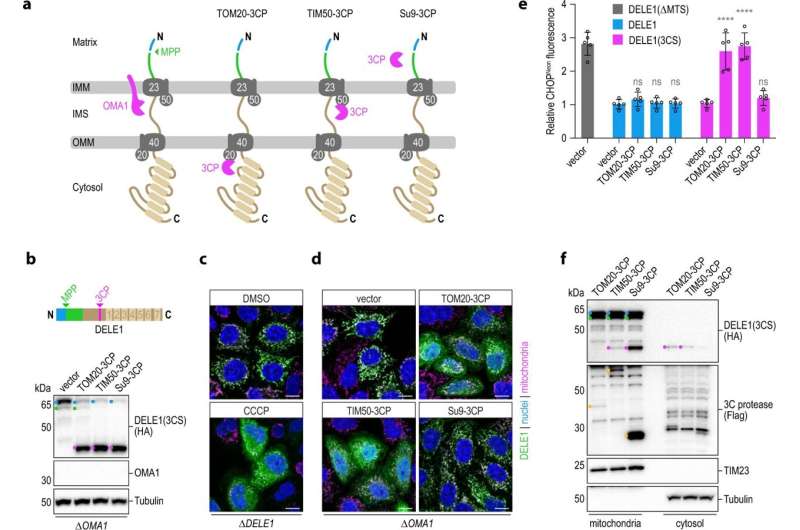
Two years ago, Jae's team explored the question as to how mitochondrial stress is actually reported to the cell. The researchers found a new signaling pathway consisting of the proteins OMA1, DELE1, and HRI, which looks after such tasks. "So we knew which factors recognize mitochondrial stress, but we didn't understand key aspects," recalls Jae. "How does the DELE1 signal travel from the mitochondrion into the cytoplasm of the cell? And how can DELE1, as an individual protein, detect the many different types of stress?"
Now the researchers have found answers. DELE1 is continuously imported into the mitochondria and processed by proteases. Deep inside the mitochondria, DELE1 is then quickly degraded. As such, there are molecules constantly passing through the outer and inner membranes of mitochondria in order to be imported.
Mitochondrial stress causes this importing process to fail. New DELE1 molecules are arrested on their way into the mitochondria and, depending on the source of the disturbance, are either cut by OMA1 or remain uncleaved outside the organelles. In any case, the portion of the DELE1 protein that possesses the signaling effect is unmasked in the cytosol. "All the different types of stress lead to one of the sub-steps involved in the importing and processing of DELE1 coming to a halt," summarizes Jae. This is how mitochondrial stress is detected.
DELE1 also recognizes dysfunctions in the mitochondrial enzymes PITRM1 and MPP. In neurodegenerative diseases, these enzymes are mutated. "Specifically in connection with such defects, we have observed that it's important for cellular survival for DELE1 to detect the problem and inform the cell," notes Jae.
What happens next? "Now that we understand the mechanism, we can investigate many different scenarios," reports Fessler. The researchers want to discover how the decision is made as to whether a cell enters a repair phase due to a stress response or goes into programmed cell death, because otherwise it would present a danger. They also hope to be able to modulate the signaling pathway such that it favors cellular survival in times of mitochondrial stress: a possible approach for treating neurodegenerative diseases.
More information: Evelyn Fessler et al, DELE1 tracks perturbed protein import and processing in human mitochondria, Nature Communications (2022). DOI: 10.1038/s41467-022-29479-y
Journal information: Nature Communications
Provided by Ludwig Maximilian University of Munich