Flux-reducing tendency of Pd-based membranes employed in butane dehydrogenation processes
Alkene production by the catalytic dehydrogenation of light alkanes is an alternative to conventional heavy hydrocarbon cracking. Dehydrogenation is an endothermic equilibrium-limited reaction and is typically performed at elevated temperatures at close to atmospheric pressure. Even at 500°C, the thermodynamic equilibrium conversion for propane dehydrogenation is less than 20%. Further, the high operating temperature results in large amounts of carbon deposition on the catalyst, which implies the need for a periodic regeneration of the catalytic bed, leading to a complex plant design. Due to the removal of hydrogen from the reaction, membrane reactors have the potential to provide the same conversion and yield of a conventional process while operating at milder conditions.
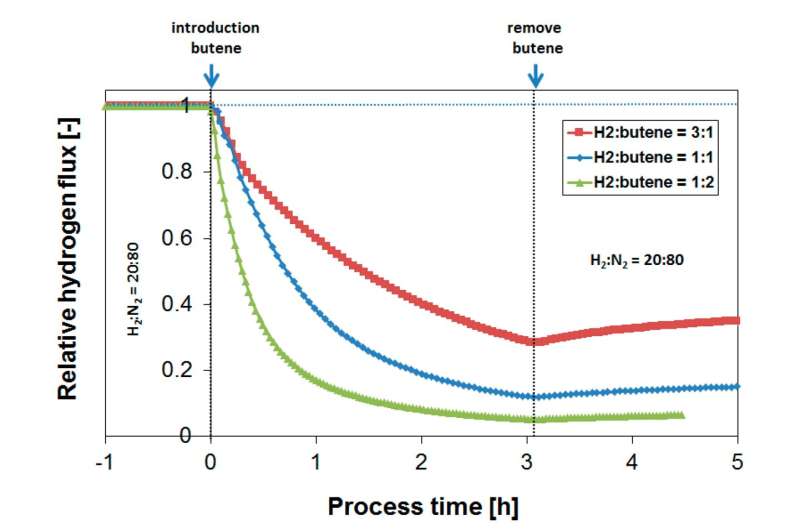
In a study published in the journal Membranes, a wide range of operating conditions, such as temperature (200–450°C) and H2/butylene (or butane) ratio (0.5–3), on the flux-reducing tendency were investigated: an optimal performance was found at 250–300°C for obtaining the highest absolute hydrogen flux in the presence of butylene. At lower temperatures, the competitive adsorption of butylene over hydrogen accounts for a large initial flux penalty.
The BIZEOLCAT project addresses the need for lowering the carbon footprint of the refining industry in a sustainable economy approach. It will develop four new processes and membrane reactors to convert alkanes (methane, propane and butane) into olefins (propylene, butadiene) and aromatics.
Petroleum, or crude oil, is not only the naturally occurring liquid hydrocarbon that is refined into fuel. It is also the starting point for many other industrially and societally important carbon-based chemicals broadly referred to as petrochemicals. Petrochemicals include common compounds such as ethylene, propylene and benzene used to make electronics, adhesives, paints, textiles, pharmaceuticals and food packaging. The BIZEOLCAT project is developing a sustainable and cost-effective process to bring the use of light alkanes (methane, propane and butane) into petrochemicals. The multinational consortium that includes expertise in research and development, industry and standardization is already planning a joint venture to ensure the work continues after the project finishes.
More information: Thijs A. Peters et al, Flux-Reducing Tendency of Pd-Based Membranes Employed in Butane Dehydrogenation Processes, Membranes (2020). DOI: 10.3390/membranes10100291
Provided by European Research Institute of Catalysis