Credit: Wikipedia
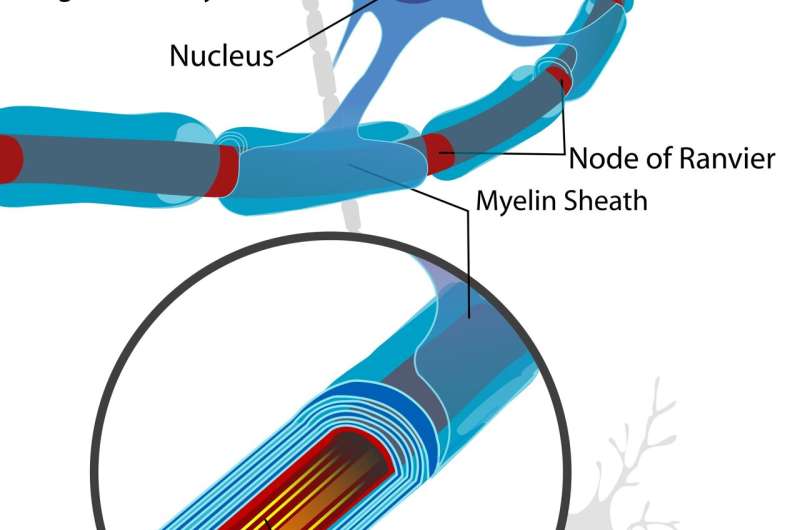
A viable molecular explanation for the origin of compact myelin of vertebrates has been a long time coming. While many invertebrates are certainly capable of wrapping their axons with crude glial extensions, none can manage anything like the massive spiral elaborations of crystalline proteolipid membrane found in the oligodendrocytes of the central nervous system and the Schwann cells of the peripheral.
Functional retrotransposons have been progressively implicated in all manner of things neurobiological. The maintenance of stem cell identity and mosaicism, incidence of neurological diseases and fusion of cells in the brain by sundry spike proteins are all now understood to be jobs for transposable elements. Writing in the bioRxiv preprint server, researchers have now discovered that vertebrate myelin likely originated when retrovirally derived elements inserted in the genome at key positions to trigger massive expression of their signature protein, Mbp (myelin basic protein).
Mbp seems to have appeared concurrently with the emergence of jaws and myelin, and is found in most ancient living vertebrates tracing all way back to the Chondrichthyes (cartilaginous fish); however, not in the Agnatha (jawless fish). It seems plausible that oligodendrocytes evolved by co-opting existing machinery from neurons or astrocytes for creating neurite-like branching structures and combining it with the specialized protein and lipid synthesis machinery from Schwann cells to construct myelin instead of synapses at their terminal ends. In this scenario, Schwann cells would necessarily have evolved first in order to speed up or otherwise power sensorimotor nerves involved in rapidly sensing, snapping or escaping. The researchers have only looked at oligodendrocytes so far, and will probably (hopefully) have more to report on the Schwann cell story.
The key element they discovered using a retrotransposon-specific rat Affymetrix Chip is a virally-derived integrase known as RNLTR12-int. Its consensus sequence is annotated as an internal sequence of endogenous retrovirus family ERV1, a relatively common group that now makes up around 3 percent of the entire genome. RNLTR12-int is just a remnant of the classic Gag-Pol ORFs (open reading frames) which is required for provirus replication cycle; It lacks the usual long-terminal repeats (LTRs) that often bookend these particular elements, and likely serves now as a long, non-coding RNA.
To prove this, the researchers first searched for and found elevated expression of RNLTR12-int-like sequences, now whimsically dubbed "retromyelin," in the oligodendrocytes of a broad panel of vertebrate subjects. They then demonstrated that this retroviral endogenization is the presumptive key step in the emergence of vertebrates by showing upregulation of Mbp through direct binding of its encoded RNA to SOX10, the signature transcription factor for myelination. A curious feature of transposable element immigration into the genome is their anchor-baby style of proliferation: These elements contain insertion sequences inside themselves, and therefore, once they manage to break into a bona-fide insertion sequence at some place in the genome, additional foreign elements related to them will automatically be afforded easy residence in their midst. That is how transposable elements of all types now make up over half our genome despite numrous efforts to molecularly suppress them them.
Most of the initial insertion events would go unnoticed, however, once a retrovirus carrying RNLTR12-int-like sequence randomly integrated into the germline, either during a primary infection event; alternatively, it could become inherited by somatically accessing the germ later via transfer from other cells through blood or nerve. Propagating stealthily by reverse transcription and re-integration, these sequences eventually chanced upon the regulatory regions for the incipient Mbp takeover whereupon it was fixed for posterity after proving itself. All the other myelin proteins like MAL, MAG, CNP, PLP, PMP22,TSPAN2 are much evolutionarily older and well-established in non-myelinated jawless vertebrates and invertebrates. More typically, what is seen after integration is that viral functionality inexorably degenerates, and the sequence contracts into a core remnant that transcribes to miscellaneous RNAs of debatable repute.
To give some further context of what this all may mean, it may be useful to induct the larger role of myelin in the vertebrate axonal ecosystem into a greater sphere of thought where additional detail might be explored. We recently discussed ATP generation in myelin within the context of potential extra-mitochondrial oxidative phosporylation. But this is not the only way for axons to get the power they need. In fact, it was just discovered that neurons can call for and get supplementary mitochondria from neighboring cells to relieve an energetic shortfall or oxygen deficit subsequent to injury. The exact method preferred by the far-reaching and uniquely bipolar dorsal root ganglion sensory neurons is to flag down CD200-expressing M2 macrophages using neuronal Isec1 receptors to engage their own complementary CD206 receptors, ultimately leading to a dispatch of vesicles filled with mitochondria.
An alternative method, used by myeloma cells to acquire mitochondria from bone marrow stem cells, is to send a retrograde signal consisting of CD38 molecules to direct construction of a nanotube highway built from various cytoskeletal proteins to transport mitochondria. The 'CD' in these proteins stands for cluster of differentiation, and traditionally, they were discovered as immune cell surface markers. These proteins are often glycoproteins, and may contain various incarnations and repeats of the original immunoglobulin domains famously employed in antibodies. Through the course of evolution, many of them were adapted to moonlight as adhesion molecules in the nervous system in order to direct partner selection at synapses, specificity of axon fasciculations, or determine the thickness and spacing in the layers of spiral glial wrappings in myelin.
The CD206 marker is a C type lectin that can act as a mannose receptor on macrophages. This receptor recycles continuously between the plasma membrane and endosomal compartments in a clathrin-dependent manner. One might infer, or at least soundly speculate, that this action could create membrane disturbances of sufficient magnitude for opportunistic vesicles laden with mitochondria to find a place to land and colonize. Several intriguing questions are presented by these kinds of behaviors. For example, do neurons, even healthy and normally active neurons, have inherent limitations in their ability to create mitochondria? Limitations on the ability to access required nuclear-encoded mitochondrial proteins invariably arise from inherent constraints on gene expression dictated by the eclectic protein needs needs of neural cell differentiation. Similarly, might simple dish experiments find that mitochondria on the move are capable of a directed oxygen mitotaxis, perhaps akin to known chemotactic behavior of egg mitochondria in response to sperm fusion in mice?
Incidentally, many instances of cell differentiation might now be re-envisioned as yet another initially breathless, but later fortuitous byproduct of transposable element insertion in promoter regions to activate genes that would induce in cells a more hospitable environment for the element to proliferate. The diverse deep molecular associations between neurons, glial and immune cells might further suggest axons may have initially enticed blood-derived cells or appropriately equipped microglial cells to immunologically protect them via immunoglobulin-related cell adhesion molecules and cytoplasmic viral defense molecules, in particular, those of the Toll and related molecule families that can sense and recognize nucleic acid segments of specific length or content. Then, through deep evolutionary time, these protector cells were converted into dedicated myelin wrapping agents genetically imprinted to permanently nourish the axons.
More information: Tanay Ghosh et al, A retroviral origin of vertebrate myelin, bioRxiv (2022). DOI: 10.1101/2022.01.24.477350
© 2022 Science X Network