Principle of production of structural colors from nematic clay double layers (DBLs). (A) Schematic of the 2D lamellar structure of synthetic Na-fluorohectorite (Na-FHt). Na-FHt spontaneously forms nematic phases of single 1-nm-thick nanosheets [single layers (SGLs)] when immersed into water. (B) Schematics of protocol for production of nematic phases of double 2-nm-thick layers (DBLs). (C) Structural colors obtained from SGL aqueous suspensions at zero ionic strength. (D) Structural colors from DBL aqueous suspensions at zero ionic strength. The clay concentrations are given in volume %. (E) Principle of reflective structural coloration obtained from a lamellar Bragg stack suspension. Each lamella is semitransparent, reflecting part of the incoming white light that then interferes constructively according to Bragg-Snell’s law, thus enhancing a single color that is both dependent on the layer distance and the angle of observation (iridescence). A dark background absorbs the white light that is transmitted through the whole stack. Only the DBL case is shown in the sketch. Credit: Science Advances.
In a new report now published on Science Advances, Paulo H. Michels-Brito, and a team of researchers in physics, inorganic chemistry, and physical chemistry in Germany and Norway, showed how bright non-iridescence structural coloration could be easily and rapidly achieved from two-dimensional nanosheets of clay mineral.
Structural colors can originate from clay mineral nanosheet solutions via constructive interference of light after reflection and scattering from nanostructures with periodicity comparable to visible light wavelengths. The scientists improved the brightness enormously by using double clay nanosheets to optimize the clay refractive index that can otherwise hamper structural coloration from such systems.
By varying the clay concentration and ionic strength, the structural colors could be precisely and reproducibly regulated to readily obtain non-iridescence. Such clay-design nanosheets can be embedded in recyclable solid matrices to simultaneously provide tunable, vivid colors, mechanical strength and stability to open a previously unknown region for sustainable colors.
Structural coloration in nature and in the lab
Structural colors result from photonic waves that interfere constructively after reflection and scattering from nanostructures with distances comparable to wavelengths of visible light. The mechanism of structural coloration is fundamentally different from the absorbance of dyes or pigments. For instance, with structural colors the material could be semi-transparent, where the color spectrum may be tuned by adjusting the nanostructures.
This mechanism can be combined with light-absorbing dark pigments as noted with major biological coloration mechanisms seen in nature; featured on birds, marine animals, some mammals, insects and certain plants. The concept of structural coloration has also sparked enormous interest in the industrial sectors, including L'Oréal's photonic cosmetics and Morphotex to represent bioinspired designs.
However, the abundance and time taken to fabricate the concept are major limitations for upscale industrial applications. Structural coloration relies on iridescence, for example the feathers of bluebirds and butterflies can be mimicked using colloidal particles. In this work, Michels-Brito et al. devised a method to produce structural colors from nematic clay double layers (DBLs). The team chose synthetic sodium-fluorohectorite (Na-FHt) – a synthetic clay mineral with superior quality relative to structural homogeneity, narrow charge distribution and a large aspect ratio, which the team characterized as materials properties.
Tunability of strutural color by adding water in the suspenstion. In half of the quartz cuvette is inserted a clay double layer suspension with a light blue color. The other half is filled with water. After mixed with the syringe needle, the sample presented a wide range of structural color. This diversity of structural colors are a result of the incomplete homogenization of the suspension, which resulted in regions with different concentrations across the sample inside the cuvette resulting in a broad range of structural colors. Science Advances, 10.1126/sciadv.abl8147
The experiments
The researchers tuned the Na-FHt to water ratio and nanosheet separations based on the wavelength range of visible light, where the photonic Bragg stacks covering the whole spectrum of colors could be produced rapidly and easily. The suspended single layers gave rise to smooth and bright colors. However, the team could improve the brightness and noniridescence of the structural colors by applying double layers (DBLs) of two suspended single layers pinned together.
As a direct biomimetic analog to this mechanism, Michels-Brito et al. compared the Loliginid squids, due to their capacity to tune their structural colors via osmotically driven changes. Structural coloration from the DBLs (double layers) relied on strong electrostatic repulsion between cofacial (lego-like) clay nanosheets to separate them to various distances by simply adding the right amount of water and choosing the wavelength that interferes constructively.
The scientists described the constructive interference of white light from individual nanosheets by using the Bragg-Snell's Law. Accordingly, the observed color depended on the layer distance and the angle of observation (iridescence). The team regulated nanosheet separation by tuning the clay concentration in suspensions in flat quartz cuvettes with a 1 mm path length to show the possibility of rapidly tuning structural colors by adding water into the solution.
-
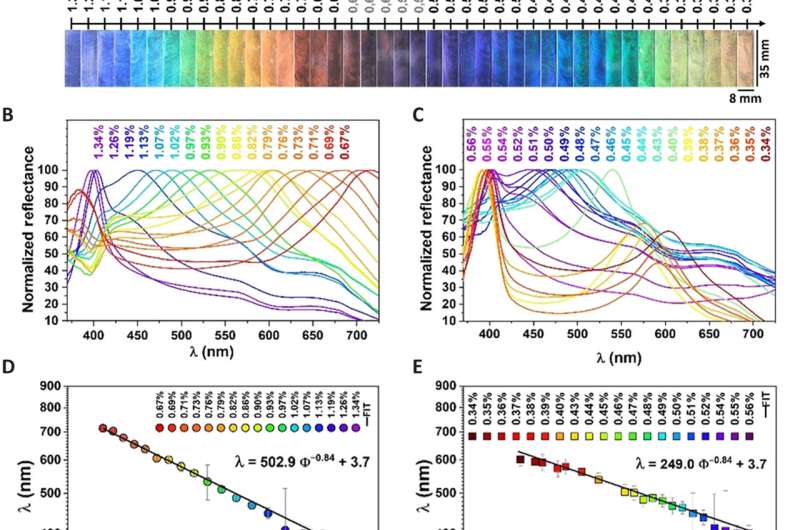
Characterization and control of structural colors from nematic clay DBLs. (A) Structural colors of the R1 and R2 ranges (fig. S6 shows the birefringence). (B) RSP for R1 range. (C) RSP for R2 range. (D) RSP maxima (with error bars) versus volume % and the linear fit. (E) RSP maxima (with error bars) versus volume % and the linear fit. Details of how the RSP maxima were determined and how the errors were estimated from these fits are explained in fig. S7. (F) d-Spacing (with error bars) versus volume % obtained from R1 and R2 ranges and linear fit. (G) RSP maxima versus ionic strength and corresponding observed structural colors. (H) CIE (Commission Internationale de l’Elcairage) diagram of the first-order colors. (I) Effect of dark and white backgrounds, respectively. Credit: Science Advances.
-
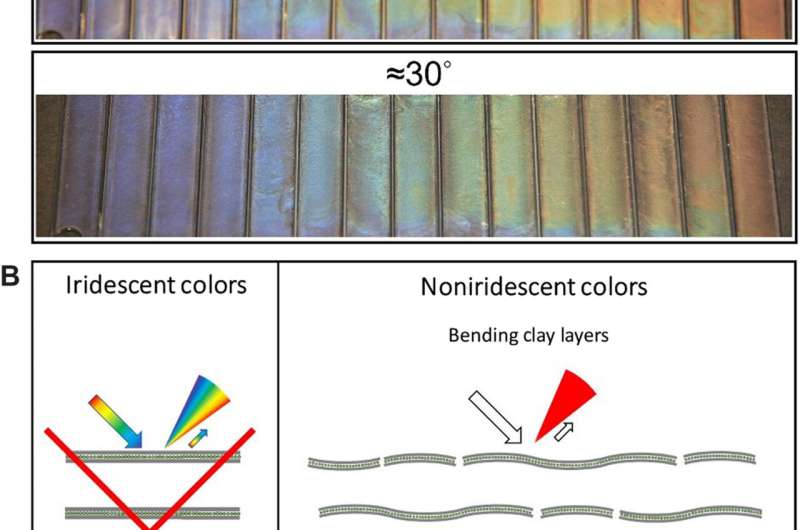
Noniridescent structural colors from nematic clay DBLs. (A) Structural colors at different angles (5° and 30°). (B) Sketch of structural order that would give iridescent colors and sketch of possible factors of disorder that, in combination, may explain the observed noniridescence color. Credit: Science Advances.
Optimizing the technique for industrial applications
The double-layer presented two different structural color changes, where the effective refractive index could be determined using small-angle X-ray scattering and reflective spectrophotometer data. Since electrostatic interactions governed nanosheet separation, the colors could be tuned by varying the ionic strength.
For example, by increasing the ionic strength of a red double layer solution, the team could blue-shift the structural color due to decreased nanosheet separation, owing to increasing electrostatic screening. During the study, all samples unexpectedly appeared non-iridescent to the eye. After close inspection, they noticed slight differences in the brightness of the colors based on the angle of view. The noniridescence of the nematic clay solutions resulted from a combination of local disorders relative to nanosheet bending and wrinkling, and turbostratic organization in the plane of nanosheets.
Michels-Brito et al. studied the samples in fixed space quartz cuvettes where sealed samples sitting 'on the desk' for more than four to five days showed some iridescence. For samples prepared in saline, such degradation times were shorter in the order of two days due to sedimentation of the solutions, which modified the colors. The team rapidly recovered the colors by gently shaking the cuvettes. These timescales of two-to-five days provided sufficient gaps to fix the noniridescent nature of structural colors in a transparent matrix for subsequent industrial roll to roll processing for pigment fabrication. The films can be reduced in thickness below 1 mm to form colors in 200 µm thick solutions.
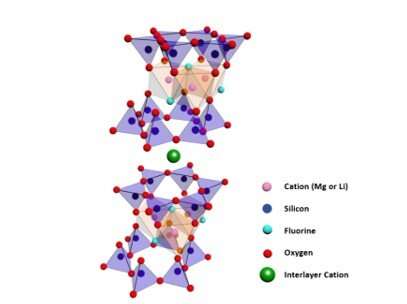
Na-Fluorohectorite structure. The orange octahedral sites (pink sphere) contain magnesium partially substituted by lithium. The octahedral sheet is sandwiched in between the blue tetrahedral sheets. The tetrahedral sites (dark blue spheres) contain silicon. The light blue spheres are fluorine, and the red spheres are oxygen. The green spheres are the interlayer cations, typically Na+ from the synthesis. Credit: Science Advances.
Outlook
In this way Paulo H. Michels-Brito et al. presented a system that accounted for the sustainability and abundance of clay minerals for upscaled applications across various areas ranging from pigments in cosmetics to healthcare, as well as windows and tiles. The outcomes of this study on synthetic clay can be transferred to natural clays, where vermiculite presents itself as the most suitable candidate to upscale the concept.
The team envision including exfoliated clay nanosheets in small amounts to polymer matrices, including biodegradable biopolymers and hydrogel matrices for structural enhancement to tune the mechanical strength and stability of the resulting composites. The results have high impact in cosmetics and personal care applications to form more sustainable and recyclable formulas, to also achieve the goals of a circular economy.
More information: Paulo H. Michels-Brito et al, Bright, noniridescent structural coloration from clay mineral nanosheet suspensions, Science Advances (2022). DOI: 10.1126/sciadv.abl8147
Minxiang Zeng et al, Iridescence in nematics: Photonic liquid crystals of nanoplates in absence of long-range periodicity, Proceedings of the National Academy of Sciences (2019). DOI: 10.1073/pnas.1906511116
© 2022 Science X Network
![Principle of production of structural colors from nematic clay double layers (DBLs). (A) Schematic of the 2D lamellar structure of synthetic Na-fluorohectorite (Na-FHt). Na-FHt spontaneously forms nematic phases of single 1-nm-thick nanosheets [single layers (SGLs)] when immersed into water. (B) Schematics of protocol for production of nematic phases of double 2-nm-thick layers (DBLs). (C) Structural colors obtained from SGL aqueous suspensions at zero ionic strength. (D) Structural colors from DBL aqueous suspensions at zero ionic strength. The clay concentrations are given in volume %. (E) Principle of reflective structural coloration obtained from a lamellar Bragg stack suspension. Each lamella is semitransparent, reflecting part of the incoming white light that then interferes constructively according to Bragg-Snell’s law, thus enhancing a single color that is both dependent on the layer distance and the angle of observation (iridescence). A dark background absorbs the white light that is transmitted through the whole stack. Only the DBL case is shown in the sketch. Credit: Science Advances. Bright, noniridescent structural colors from clay mineral nanosheets](https://scx1.b-cdn.net/csz/news/800a/2022/bright-noniridescent-s.jpg)