Credit: Wikipedia commons
One of the biggest challenges in biology today is to explain the structure of cristae, the inner membranes of mitochondria. An explanation in this case is a set of principles to predict what form the cristae will take after basic metabolic manipulations of the environment the mitochondria are in. These principles will therefore be a description of the true function of mitochondria, something that has so far only been scarcely imagined.
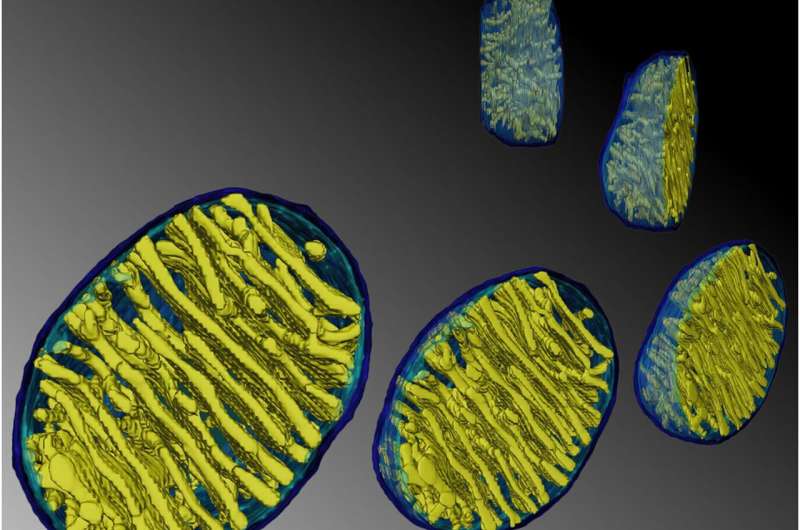
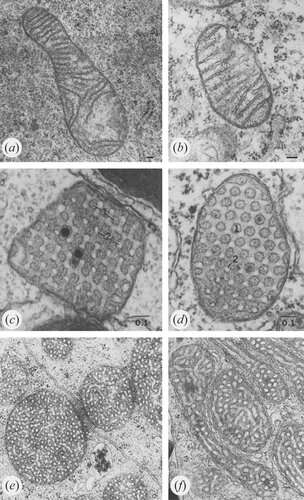
Recent advances in techniques like live cell super-resolution light microscopy and electron tomography have given new insight into the dynamic behavior of cristae. A detailed architecture of the entire mitochondrial volume can now be constructed from a series of tilt images that are back-projected to create 3D tomograms. On Monday, we discussed how cristae are morphed and reconfigured according to the abundance and health of several inner membrane and matrix proteins. The pretext for that analysis was the structural and biochemical similarities between membranes in mitochondria, thylakoids, and myelin that presumably help channel metabolites in the production of energy.
In a recent article from The Royal Society's Open Biology, researchers explain the biogenesis of cristae through the coordinated activities of four major pathways that are evolutionarily preserved going from protists and yeasts up to higher eukaryotes like ourselves: dimer formation and oligomerization of the ATP synthase at cristae rims, assembly of the 'mitochondrial contact site and cristae organizing system' (MICOS) at crista junctions, membrane remodeling by an inner-membrane-associated, dynamin-related GTPase (Mgm1 in yeast and OPA1 in mammals) and proper adjustment of the membrane lipid composition.
For the first pathway involving the ATP-synthase, several things are apparent. As we reported earlier, the spontaneous dimerization of ATP-synthase at precisely defined and species-dependent angles into ordered rows dictates the ground-floor geometry. In contrast to respiratory complexes I-IV, which are assembled on the flat inner boundary membrane, ATP-synthase (complex V) is fully assembled deep in the cristae membranes. While several ATP-synthase subunit proteins are dispensable for proper cristae formation, the Atp20 and Atp21 subunits are strictly required.
An excess of ADP induces a condensed conformation with large, swollen intra-cristal spaces. By contrast, under ADP-limiting conditions, mitochondria adopt the orthodox conformation with contracted intra-cristal space. In the giant amoeba Chaos carolinensis, mitochondria normally contain randomly oriented tubular cristae. With starvation, enlarged cristae adopt a cubic morphology with a zigzag-like pattern. In mice, apoptotic agents cause fusion of individual cristae with subsequent release of cytochrome c from the intra-cristal space into the boundary region.
Credit: Klecker and Westermann, Open Biology (2021). DOI: 10.1098/rsob.210238
For the second pathway, the assembly of MICOS contact sites, research has determined critical proteins such as those from the MIC60-related gene family are present as far back as the endosymbiotic ancestors of mitochondria—the α-proteobacteria. Many of these earlier mitochondrial forbearers already show differentiated intracytoplasmic membrane structures. Invariably, species that have simplified their mitochondria to the point that cristae are absent correspondingly lack the MICOS-related genes. Re-expression of MIC60 homologues into Δmic60 yeast mutants rescues the ultrastructural mitochondrial defects.
The third pathway includes the dynamin-related GTPases, which coordinate fusion and fission of both inner and outer membranes. In fission, these proteins polymeize into contractile rings that apply constrictive forces to squeeze mitochondria. The outcome is now understood to be dependent on interactions of these proteins, both with the MICOS complex and cristae junctions, and also the inner and outer membrane transport systems that congregate there. These include the TIM and TOM membrane translocator complexes.
The fourth pathway comprises the mitochondrial membrane phospholipids themselves. Mitochondria harbor the cardiolipin biosynthetic pathway, and are also involved in the synthesis of phosphatidylethanolamine. Together with phosphatidylcholine, these are the three major phospholipids mitochondria are working with. Most of the building blocks for mitochondrial lipids are synthesized in the ER and must therefore be imported in by mechanisms involving close apposition to the ER. Once inside the outer membrane, the distribution of lipids is mediated by intermembrane-space-localized transport proteins of the Ups/PRELI family,
Mitochondria don't create geometry from scratch, but rather harness and build upon the natural physical forms that occur spontaneously in lipids. Left to their own devices, lipids form concentric lamellar structures which can then be expanded and augmented by specific proteins. Fine-scale measurements have now revealed that individual cristae are functionally independent and can have significantly different membrane potentials.
Cristae formation involves a tightly connected interplay of the above four shaping influences. For example, the activities of the MICOS complex and ATP synthase dimerization are both cooperative and antagonistic. MICOS induces negative membrane curvature while the ATP synthase induces positive curvature at cristae tips and rims. New computational models, as currently under development in labs throughout the world, where ratios of these different components can be finely tweaked and adjusted, will greatly aid in defining what controls the form of mitochondria.
More information: Till Klecker et al, Pathways shaping the mitochondrial inner membrane, Open Biology (2021). DOI: 10.1098/rsob.210238
Journal information: Open Biology
© 2022 Science X Network