Credit: F. Pacheco-Vázquez, R. Ledesma-Alonso, J. L. Palacio-Rangel, and F. Moreau, https://journals.aps.org/prl/abstract/10.1103/PhysRevLett.127.204501
If you've seen water drops dance and jitter on a hot pan or griddle, you've seen the Leidenfrost effect in action. Or you may have seen the "Mythbusters" episode where Adam and Jamie thrust their wet fingers and hands into molten lead and pulled them out unharmed.
The effect relies on the finger being wet, so it has a film of water on and around it. In molten lead, that water film boils, creating steam, which is a poor conductor of heat. That gas, which is water vapor, insulates the finger long enough to protect it for a short period of time when dipped into the molten lead, at 328 degrees Celsius (622 degrees Fahrenheit) or higher.
Likewise, a water drop on a hot plate evaporates at its bottom edge, creating a insulating cushion that keeps the drop levitating as a liquid for a surprising amount of time. It was first described by German doctor Johann Gottlob Leidenfrost in 1751.
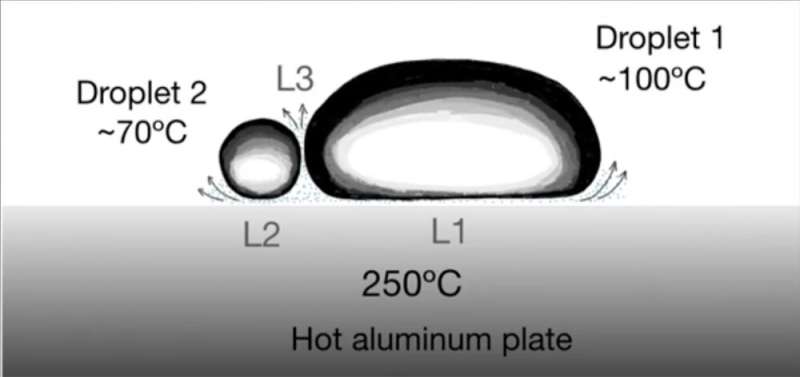
Now, a group of scientists from Mexico and France have for the first time published the results of experiments showing that two hot drops of different liquids can also bounce off one another due to the Leidenfrost effect between them. The group calls this a triple Leidenfrost effect, since both drops are already on a hot plate experiencing their own Leidenfrost effect with respect to the plate, and an additional Leidenfrost effect when they collide with and bounce off one another, developing a third vapor cushion at the collision interface between the drops.
In the experiments, the hot aluminum plate had a slightly concave top surface to keep the droplets toward the center of the plate. For water droplets 0.5 ml in volume (0.5 cc), the droplets entered a Leidenfrost state at a plate temperature of 210 degrees Celsius. At that point, the droplet lasted about 450 seconds (7.5 minutes) due to water's large latent heat (the amount of heat required to change water from a liquid to a gas at constant temperature). After that, the droplet evaporated completely and was gone, turned to water vapor.
Other liquids had different Leidenfrost temperatures and duration times: Ethanol droplets entered the Leidenfrost state at about 150 degrees Celsius and lasted about 200 seconds, and chloroform at about 150 degrees Celsius for 100 seconds. The research was conducted in Puebla, Mexico, at about 2,200 meters (7,218 feet, 1.37 miles) above the sea level, where, for example, the boiling point of water was only 93 degrees Celsius (199 degrees Fahrenheit). Other thermodynamic properties might have similar adjustments.
A small blue droplet of ethanol on a hot aluminum plate repeatedly bounced off a larger, clear droplet of water, exhibiting three different Leidenfrost effects at the same time. Eventually the blue droplet decreases in size and becomes spherical and its vapor layer can become evacuated, and the droplets coalesce. Credit: F. Pacheco-Vázquez, R. Ledesma-Alonso, J. L. Palacio-Rangel, and F. Moreau, https://journals.aps.org/prl/abstract/10.1103/PhysRevLett.127.204501
After the researchers determined the Leidenfrost temperatures for 11 low viscosity liquids, each with different boiling temperatures, they deposited two droplets of different materials on the hot aluminum plate with a temperature of 250 degrees Celsius (482 degrees Fahrenheit). Each droplet underwent its own Leidenfrost effect with a vapor layer underneath it, levitating as it made its way down toward the center of the plate. Near there, the levitating droplets would collide.
At that instant, one of two things happened: The droplets either coalesced or they bounced off one another.
Coalescence happened in milliseconds if the liquids were of the same substance, such as water-water, or if they had similar properties, for example, ethanol-isopropanol.
In the more interesting cases, the droplets bounced off one another. This happened when the droplets were of different liquids, for example, water-ethanol or water-acetonitrile. Each levitated from its own Leidenfrost effect. But a vapor cushion also surrounded each droplet on its side, so as the droplets collided, there was a vapor cushion there that prevented a merger of the droplets. In fact, the rebounding velocity of a droplet could sometimes be larger than its impacting velocity, because the pressure in the vapor layer between the droplets was enhanced by both droplets' Leidenfrost layer. This same vapor layer is what prevented initial coalescence.
The smaller droplets bounced repeatedly off the larger droplet over several seconds, sometimes minutes (see video above). Eventually, the smaller droplet changed from a pancake shape to a spherical shape, when its vapor layer was evacuated during the collision time and the drops finally coalesced. Filming the process at high speed revealed that the diameter of the smaller droplet decreased linearly with time before coalescing.
Only two parameters determined the conditions for direct coalescence or bouncing: the difference in surface tensions between the liquids (surface tension is an inherent property of a liquid, measured in force per unit length) or the difference in boiling temperatures. When the difference in boiling points was large, the smaller droplet could explode violently, as in glycol-chloroform.
Other dynamics based on the Leidenfrost effect have been explored in recent years, such as self-propulsion of droplets, sustained rotations, oscillations and exploding droplets, suggesting the possibility of manipulating the Leidenfrost effect on droplets for applications in engineering and microfluidics. This current work of understanding how Leidenfrost drops of different liquids interact adds another dimension to potential applications.
More information: F. Pacheco-Vázquez et al, Triple Leidenfrost Effect: Preventing Coalescence of Drops on a Hot Plate, Physical Review Letters (2021). DOI: 10.1103/PhysRevLett.127.204501
Journal information: Physical Review Letters
© 2022 Science X Network