Missing 'key' could overcome drug discovery barrier
Scientists have found a missing 'key' that unlocks critical channels responsible for potassium ions to flow across cell membranes in a process that is essential for life. The discovery overcomes a major barrier to the development of novel drugs targeting a host of diseases, including some cancers.
The research team from WEHI and La Trobe Institute of Molecular Sciences (LIMS) have identified the 'key' to opening a molecular gate controlling currents of potassium ions across cell membranes. Ion currents transmit nerve signals in the brain and nervous system, regulate the heartbeat, and facilitate a host of critical cellular and tissue processes.
Dysregulation of ion channels has been implicated in the development, progression and spread of some cancers, as well as neurological, cardiac and kidney disorders including epilepsy and diabetes. Sadly, while ion channels are widely considered to be important druggable targets, it has proved difficult to exploit them.
The research, led by Dr. Jacqui Gulbis from WEHI and Professor Brian Smith from La Trobe University, resolves a decades-old problem, making a conceptual breakthrough in understanding how the channels are gated. The study was published in the journal Nature Communications, with first authors Dr. Ruitao Jin and Sitong He from LIMS and Dr. Katrina Black from WEHI.
Myth-busting
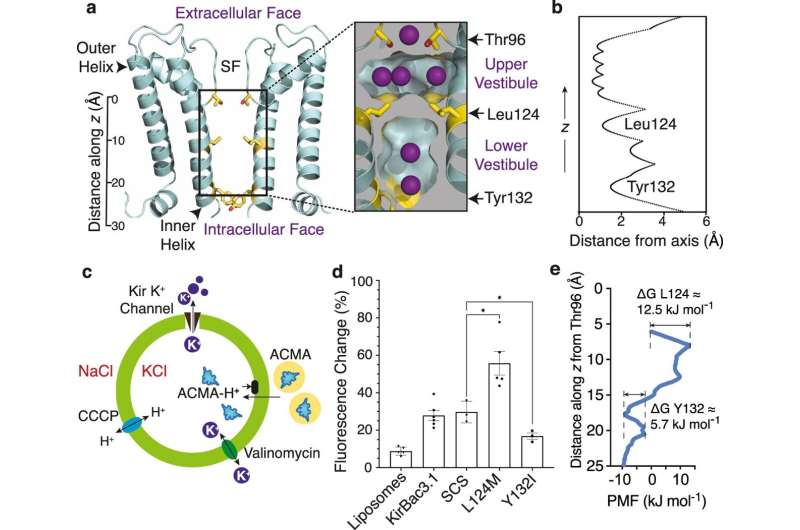
Potassium ion channels are tiny, gated, pores in cell membranes that permit controlled potassium flow in and out of cells. Potassium conduction is regulated by an internal gate in the channel; when it opens, potassium flows across the membrane, transmitting electrical signals essential for life.
Dr. Gulbis has been studying ion channels for around 25 years.
"Ion channels facilitate all cell biology," Dr. Gulbis said. "They set up the cellular environment and, as well as the electrical signaling that causes the heart to beat and allows nerve impulses and muscle contractions. They set off cellular signaling pathways, so conduction through these channels is tightly controlled. In some medical conditions, this goes awry. Until now, we didn't know how to even start to fix this."
A previous discovery of the team had overturned a widely accepted theory that potassium channels must physically widen to allow ions to cross the membrane, Dr. Gulbis said.
"In a paper released last year, we showed that the channel continues to function even when rendered incapable of physically widening. So, while we knew that the prevailing theory was incorrect, it is only with this new paper that we begin to explain what is really happening."
The missing key
Dr. Gulbis said the research team discovered that the cell membrane in which the ion channel is embedded held the missing 'key' that controls the flow of potassium ions.
"We applied structural biology, using data collected at the Australian Synchrotron, and other biophysical methods to show that specific fatty lipids from the membrane interact tightly with the channel to open a gate that allows potassium ions to pass through. The answer was hiding in plain sight," she said.
"There is a physical gate, but it is not located where others have thought it was. Potassium ions have their electrical charges shielded by a 'cloak' of water molecules, and for decades, scientists believed there must be a widening of the channel to allow ions, enlarged by this tightly held mantle of water molecules, to traverse the cell membrane. Last year we showed that this was not the case, demonstrating that potassium can shed some of its water molecules to pass through a very narrow opening. Disproving the conventional understanding was the first step in providing an alternative explanation for how potassium flow across membranes is controlled.
In this new study we have identified the gate, described its nature, and showed how specific membrane lipids engage with the channel to operate it. Not only is the gate in a different place than previously thought, it operates by a subtler, and completely different, process. Once you see it, it's obvious," she said.
Dr. Gulbis said that together these discoveries overturn and redirect our basic understanding of how potassium channels work.
Professor Smith said it provided evidence of how dynamic the cell membrane was.
"The cell membrane is often thought of as inert or passive, but we have shown that it is actually incredibly dynamic, and lipids that are bound within the cell membrane have a much more active role in controlling proteins and signaling than is typically considered."
Professor Smith said the team made the discovery using sophisticated computer simulations of the channels using the National Computational Infrastructure (NCI) Australia and the LIEF HPC-GPGPU Facility at the University of Melbourne.
"We used millions of hours of high-performance computing to run mathematical simulations to make this discovery, using the type of hardware that is typically used for gaming or mining bitcoin," he said.
Drug discovery
Professor Smith said the discovery should reignite the search for drugs that target ion channel deregulation to treat diseases.
"Disorders of ion channels—channelopathies—have been implicated in many conditions including cardiac diseases, neurological and nerve disorders, kidney disease and diabetes. Dysregulation of these channels has also been implicated in the progression and spread of some cancers, because it favors the tumor microenvironment," Dr. Gulbis said.
"For more than 20 years the search for pharmaceuticals that can exploit ion channels to treat disease has been in hiatus, because drug companies were laboring under a folkloric conviction as to how these ion channels work. This new information on how potassium channels are controlled—or at least the nuts and bolts of it—will open up new avenues and ideas for the rational discovery and design of new treatments."
More information: Ruitao Jin et al, Ion currents through Kir potassium channels are gated by anionic lipids, Nature Communications (2022). DOI: 10.1038/s41467-022-28148-4
Journal information: Nature Communications