January 27, 2022 feature
When fossils come to life: SARS-CoV-2 spike, syncytin-1, and other curious fusion proteins
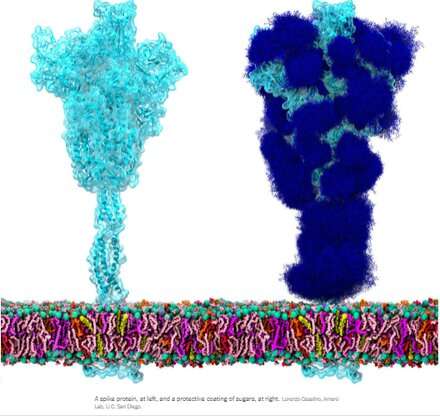
The homotrimeric spike glycoprotein (S) from SARS-CoV-2, particularly its S2 subunit, is a fusion protein extraordinaire. It can fuse viral particles to cells and also fuse cells to cells to create multifarious syncytia among different cell phenotypes. Depending on which exact versions are under consideration, the spike can perform these feats via multiple mechanisms acting at both the intracellular and extracellular sides of cell membranes.
These fusion functions are somewhat analogous to typical homotrimeric ENV (envelope) proteins like our syncytin-1 endogenous retroviral ENV protein, and the GP160 ENV glycoprotein from the HIV virus. GP160, the HIV 'spike' protein, is ultimately processed at its own furin cleavage site (also found in syncytin-1) into a GP120 and a GP41 protein, both of which can adopt distinct pre- and post-fusion configurations. The SARS-CoV-2 genome, however, already specifies a separate small ENV protein (conveniently designated as E), which assembles into a presumptive cation channel with a central fusion pore.
There are no env, pol, gag, or pro genes defined as such for the SARS-CoV-2 genome, as is the case for the retroviruses, which must integrate into our DNA as part of their lifecycle. Curiously, researchers have found that the SARS-CoV-2 spike protein, behaving of its own accord, directly participates in the activation of endogenous retroviruses in our cells which contributes to observed pathology. What exactly is going on here?
In light of some of these similarities, it has been suggested that antibodies generated against the spike protein could potentially cross react in any tissues that might express endogenous retroviral proteins. In particular, during pregnancy, whereupon placental trophoblasts express very useful ENV proteins from many such HERVs including ERVW1 (syncytin-1), ERVFRD-1 (syncytin-2), ERVV-1, ERVV-2, ERVH48-1, ERVMER34-1, ERV3-1, and ERVK13-1. Syncytin-2 expression in villous cytotrophoblasts, however, is highly correlated with the degree of severity of some placental pathology like preeclampsia.
Fortunately, researchers found that there is virtually no direct sequence homology between spike and syncytin-1, and little chance of cross reactivity. Writing in the journal Animal Cells and Systems, Korean researchers tested a large panel of monoclonal antibodies and were able to conclude that while ancient genomic relics like HERVs can be activated in various tissues by SARS-CoV-2, there is no risk of cross reactivity or even infertility.
But if spike protein is still very capable of causing undesirable cell fusion, how can we better define this seemingly random activity, and furthermore, what might we do about it? Perhaps the first step is to get a little more sophisticated with the terminology of cell fusion. Writing in the journal Oncotarget, author Yuri Lazebnik offers some food for thought. A syncytium produced from cells of the same type, for example, as in the fusion of two or more pneumocytes, is termed a homokaryon. A heterokaryon then, would be a syncytium made from say, a pneumocyte fused to an epithelial progenitor, or perhaps a leukocyte. In general, once cells combine all bets are off—whether they can later sort themselves and their nuclei to generate replication competent mononuclear offspring is still incompletely known.
Formation of spike-induced syncytia in the lungs of COVID-10 patients has been found in many forms, each with their own emergent properties that can contribute to disease sequelae. For example, ciliated cells in the airway, alveolar type 2 pneumocytes, and epithelial progenitors have all been found to participate in the oft-observed multinucleated 'giant cells.' Throw in a spike-laden leukocyte or two and things rapidly get hard to predict. Perhaps an even more alarming situation would be formation of a syncytium in the cells lining our blood vessels that could contribute to thrombosis. The ensuing death and eventual sloughing off of a patch of inappropriately fused cells could expose a sizeable region of thrombogenic basement membrane. A 20-micron fiber of collagen, the main component of the basement membrane, is sufficient to trigger platelet-dependent clotting.
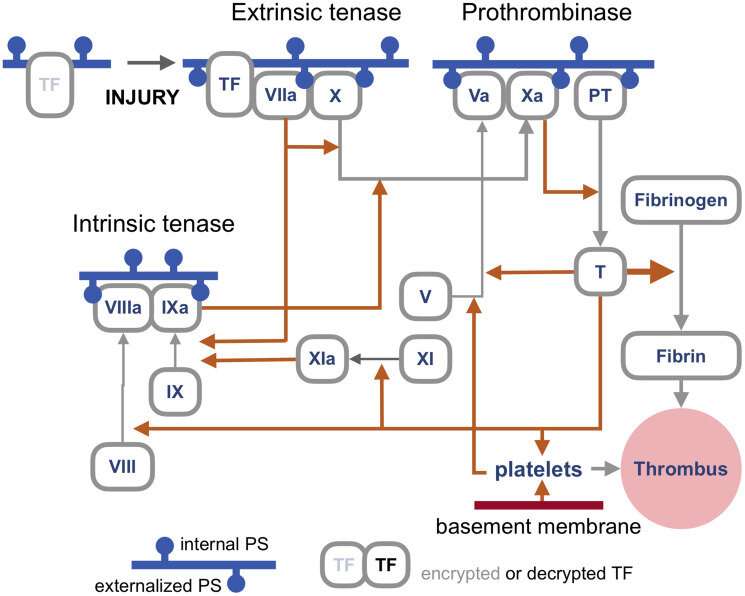
But enough of this fear-mongering. Researchers have identified a set of already approved drugs that prevent spike-induced cell fusion and inhibit TMEM16F, a critical protein for syncytium formation. TMEM16F has the dual role of being a calcium-activated ion channel that regulates chloride secretion, as well as a lipid scramblase that relocates phosphatidylserine (PS) to the cell surface. This PS externalization is required for cell fusion in many systems, including spike-induced syncytia. Incidentally, scramblases also control the rate-limiting steps of the blood coagulation cascade, and may be the mechanism behind spike-induced thrombosis.
The blood coagulation pathways are a whole separate complex can of worms, but suffice it to say here that the primary trigger of coagulation induced by viral infections is the so-called extrinsic 'tenase.' Tenases are enzymes that process Factor X, or FX, (hence ten-ase), in the Tissue Factor (TF) activation pathway, which essentially act as the fuse for thrombus generation. The resulting complexes are assembled on externalized PS in the presence of calcium ions. TF is, in a sense, encrypted and so is unable to activate its downstream target factor FVIIa until it is de-encrypted by externalized PS.
The realization that some fusogenic viral, or even retroviral proteins, which are fortuitously expressed in particular regions of the body may likely contribute to thrombotic events is of tremendous practical importance. One need look no further than Moderna's ample proposed pipeline for therapeutic mRNA-delivered amenities based on these proteins for all manner of viral insults to have some pause for inspection. There is certainly no shortage of methods currently employed for building antigenic spike proteins for vaccinations. Depending of how much of the spike code is used, which cleavage sequences are included, and which parts are stabilized, very different proteins can be made. It is probably fair to say that full-length spikes in large questionable vectors or inactivated viral particles have been largely de-emphasized in favor of smaller but more immunogenic concoctions.
Lungs and blood vessels are certainly critical areas of concern in any SARS-CoV-2 infection, but what our precious neurons—can spike fuse them as well? Clearly it can fuse neurons in brain organoids, but then again, what can't researchers do with these instant research publication miracles. In looking more braodly at other kinds of viruses, the fusion of neurons, glial cells, and even axons seems to be par for the course in causing a myriad constellation of potential neurological issues. For example, the pseudorabies virus bridges synapses to electrically couple the activity of neurons by fusing their axons. Fusions with glia have similarly been detected and linked with lasting neuropathic pain after the acute phase of herpes zoster (shingles). As of this Wednesday, we all are further aware that SARS-CoV-2 can utilize the protein vimentin as a way to infect endothelial cells. It should escape no one's notice, or at least that of the neuroscientists, that vimentin is the prime marker used for identifying glial cells.
Additionally, the rapidly expanding body of work looking at endogenous retrovirus re-activation in neurodegenerative disease further suggests to the rational mind that cell fusion may also be involved in this kind of pathology.
More information: Serpen Durnaoglu et al, Syncytin, envelope protein of human endogenous retrovirus (HERV): no longer 'fossil' in human genome, Animal Cells and Systems (2022). DOI: 10.1080/19768354.2021.2019109
Journal information: Oncotarget
© 2022 Science X Network