Graphical abstract. Credit: DOI: 10.1002/anie.202111228
In the quest for new materials with excellent properties for specific applications, scientists have developed various synthesis strategies over the years, which have given them increasingly precise control over the process and, consequently, over its outcome. In reticular chemistry, specifically designed molecular building blocks are used to create new porous crystal structures, whose properties are strongly related to the periodic arrangement of those basic components. These so-called reticular materials include metal-organic frameworks (MOFs), covalent-organic frameworks (COFs) and metal-organic polyhedra (MOPs).
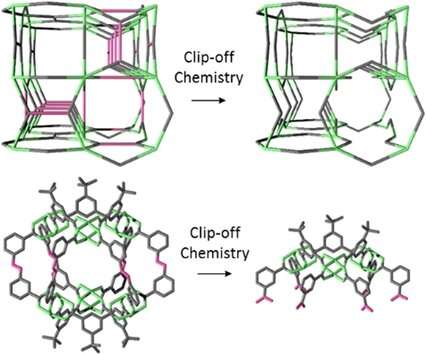
A team of researchers from the Catalan Institute on Nanoscience and Nanotechnology (ICN2), the Materials Science Institute of Madrid (ICMM-CSIC), the Universitat Autònoma de Barcelona (UAB) and the ALBA Synchrotron Light Facility (Barcelona)—coordinated by ICREA Prof. Daniel Maspoch and Dr. Inhar Imaz, respectively group leader and senior researcher in the ICN2 Supramolecular NanoChemistry and Materials Group—has pushed forward the approach to structure design of reticular chemistry by proposing a novel synthetic strategy. In what they named clip-off chemistry, new materials or molecules are generated by selectively breaking some of the bonds in known reticular materials.
In a paper recently published in Angewandte Chemie, they demonstrated the validity of their approach by modifying three-dimensional MOFs or zero-dimensional MOPs via splitting—rather than formation— of bonds in their architecture. Since only selected bonds are cleaved, the resulting structures present a new topology and different properties, while retaining its original dimensionality. For this technique to work, however, the precursor reticular material must have cleavable alkene groups (which are functional groups containing one or more carbon-carbon double bonds) located at specific positions in its structure.
Using clip-off chemistry for the first time, the authors of this work synthesized new molecular structures, starting from existing ones. In order to perform these syntheses, cleavable alkene groups were chemically introduced in the precursor materials. In practice, some of the original linkers were replaced with others of similar size and geometry containing the desired groups, that is, the breakable bonds. After appropriate treatment, those bonds split and what is left is a lattice structure made up only of the circuits of connections that did not include the cleavable groups.
A powerful new tool for forging novel materials and molecules is therefore provided to scientists. The application of such technique to a variety of structures can lead to the engineering of a plethora of new molecular blocks and structures.
More information: Yunhui Yang et al, Clip‐off Chemistry: Synthesis by Programmed Disassembly of Reticular Materials**, Angewandte Chemie International Edition (2021). DOI: 10.1002/anie.202111228
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Autonomous University of Barcelona