Modeling how cells choose their fates

It may seem hard to believe, but each one of us began as a single cell that proliferated into the trillions of cells that make up our bodies. Though each of our cells has the exact same genetic information, each also performs a specialized function: neurons govern our thoughts and behaviors, for example, while immune cells learn to recognize and fight off disease, skin cells protect us from the outside world, muscle cells enable movement, and so on.
All of these cell types have a common origin as so-called pluripotent stem cells. Full of possibility, stem cells are like a blank slate that can become any type of cell. As an analogy, think of how a child grows into an adult and chooses a career and life path. How stem cells choose their careers depends on complicated chains of reactions within a cell's genome (its DNA), called genetic circuits.
Now, researchers in the laboratory of Caltech's Michael Elowitz, professor of biology and bioengineering and Howard Hughes Medical Institute Investigator, have developed a synthetic genetic circuit that demonstrates how cells could choose their fates. The research is described in a paper that appears in the journal Science on January 20.
Using this circuit, which they termed MultiFate, the researchers showed how a relatively small set of protein components and interactions are sufficient to establish and control a larger number of cellular states through a property called "multistability." MultiFate now enables researchers to engineer a single living cell that can change into different states that are each stable on their own but are capable of performing a distinct function—analogous to what happens in our own bodies.
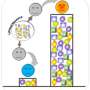
Led by graduate student Ronghui Zhu, the researchers designed an artificial circuit of genes that could function within cells grown in the lab without interfering with normal cellular processes. The MultiFate circuit consists of three genes, each coding for a corresponding transcription factor (a protein that turns on the expression of genes) labeled with a distinct color protein: red, green, or blue. Each of these three proteins turns itself on by binding to its own DNA. The three types of proteins can also stick to one another to block each other's activity.
As predicted by the team's mathematical model, this type of circuit can allow a cell to exist in as many as seven distinct states. Like pixels on a computer screen, each of these states expresses a different combination of the red, green, and blue proteins, making the cells glow in any of seven different hues: red, green, blue, cyan, white, magenta, or yellow. Once it is in one of these states, the cell remains in it unless it is deliberately perturbed by the researchers. Because the cells are locked into their fates, a cell passes its fate (color) to its daughter cells as it grows and divides.
In addition, unlike natural cellular circuits, which can be difficult to control, the researchers designed MultiFate so that they could induce the cell to switch between the seven states using certain drugs.
"This work shows how designing and building synthetic circuits from scratch can provide insights into fundamental biological phenomena. MultiFate is inspired by properties of natural cell-fate control circuits but designed from the bottom up. Not only does it help explain how cells can exist in so many fates, it also could provide a foundation for extending cell therapies to take advantage of multiple cell types to perform more complex therapeutic functions that no single cell type could provide," says Elowitz.
The paper is titled "Synthetic multistability in mammalian cells." Zhu is the paper's first author. In addition to Zhu and Elowitz, co-authors are Caltech graduate student Jesus M. del Rio-Salgado and Jordi Garcia-Ojalvo of Universitat Pompeu Fabra in Barcelona, Spain.
More information: Ronghui Zhu et al, Synthetic multistability in mammalian cells, Science (2022). DOI: 10.1126/science.abg9765
Journal information: Science
Provided by California Institute of Technology