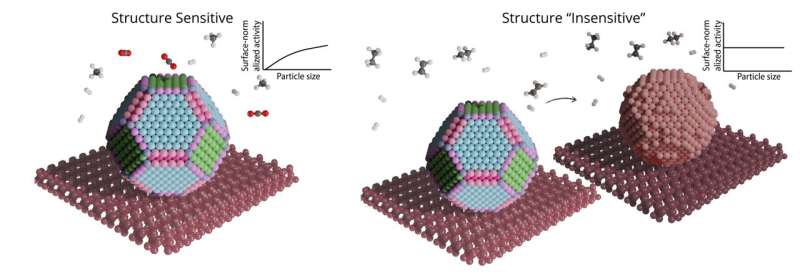
A schematic representation of the difference between structure sensitive and (apparent) structure insensitive behavior of catalytic particles. Credit: Utrecht University
An international team of researchers has solved one of the fundamental mysteries in catalysis: The paradox of structure insensitivity. To make better catalysts, researchers have long been studying the optimal shape and size of these nanoparticles, as this should have an influence on how well they perform in chemical reactions. Mysteriously, for some catalysts, the shape and size of the nanoparticles seemed to have no influence on their performance. A research team led by Prof. Bert Weckhuysen has now shown that this is not true. Instead, these supposedly "structure-insensitive" particles manage to rearrange themselves extremely quickly to an optimal structure, which is why their performance is always similar. This insight can help in the development of catalysts for cleaner fuels, for example.
The team of researchers at Utrecht University, in collaboration with Technion Institute of Technology, Eindhoven University, Oak Ridge National Laboratory, Stony Brook University, and the Paul Scherrer Institute, published their results on 7 December in Nature Communications.
Never truly explained
Catalysts are widely used in the chemical industry to more efficiently make fuels, chemicals and materials, including plastics, coatings and pharmaceuticals. Catalysts are nanoparticles, only a millionth of a millimeter in size, that work by activating molecules on their surface. Since catalyst particles are so small, their performance is highly dependent on their shape: A very small particle has more irregular surfaces with single atoms "sticking out," while larger particles have more "flat" sides of atoms.
Knowing that molecules react differently on the regular versus irregular planes of atoms, one would thus expect that larger nanoparticles behave completely differently than smaller ones. However, reactions have been observed where this does not occur. "These 'structure-insensitive' reactions were never truly explained," states Charlotte Vogt, first author of the paper.
Rapid restructuring
The researchers used a combination of advanced infrared spectroscopy, X-ray spectroscopy and electron microscopy to study a set of well-defined supported nickel catalysts and compared their activity for a structure-insensitive reaction, turning ethene into ethane, and a structure-sensitive one: Turning carbon dioxide into methane.
In the supposedly structure-insensitive reaction, they observed a rapid surface restructuring of the metal nanoparticle. This restructuring happens faster as well as more pronounced for larger metal nanoparticles than for smaller ones. The most likely explanation for empirically observed structure insensitivity is thus not the absence of sensitivity to geometrical and electronic effects, which explains structure sensitivity, but the fact that the metal surface rapidly changes as a function of particle size leaving only very specific sites exposed. Therefore, the researchers propose to replace the term "structure insensitivity" with "apparent structure insensitivity."
More information: Charlotte Vogt et al, Dynamic restructuring of supported metal nanoparticles and its implications for structure insensitive catalysis, Nature Communications (2021). DOI: 10.1038/s41467-021-27474-3
Journal information: Nature Communications
Provided by Utrecht University Faculty of Science