Unexpected chemical reaction may offer new industrial applications

Newly discovered chemical reaction involving water-soluble particle surfaces is now being presented in a new study in Science. Initiated by a team from the University of Gothenburg, this study may be useful in the future for sewage treatment, for example.
Gases and aerosol particles play important roles for the chemistry in the atmosphere. Their interactions affect clouds and the climate. Typical examples of aerosols are smoke, fog and air pollution.
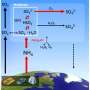
Aerosol particles are especially important, even if they are not visible, because they have surfaces in an atmosphere that otherwise consists only of gases. A new study has now found evidence that when these surfaces begin to absorb water and dissolve, new and previously undiscovered chemistry can take place.
Surprising discovery of new chemistry
In the new article in the journal Science, the researchers describe that when a surface of ammonium sulfate dissolves, it promotes sulfate-reducing ammonium oxidation.
The discovery is surprising, because this reaction ordinarily requires extra energy to overcome a barrier and therefore does not occur spontaneously.
"It was a surprise to see the reaction occur, but we came to understand that the water-soluble salt surface made the reaction possible. This has led us to rethink the catalytic effects of surfaces, where some reactions actually can be promoted in the right conditions," says Xiangrui Kong, researcher at the University of Gothenburg and lead author of the study.
Possible benefits for cost-effective industrial and environmental applications
The research has ramifications for our understanding of the atmospheric sulfur cycle and the occurrence of other important compounds involved in many chemical reactions in the atmosphere.
The sulfate-reducing ammonium oxidation reaction that was observed may also be useful for sewage treatment, for example, and other industrial applications. Biocatalysts or other expensive methods are currently required to activate such reactions. According to the researchers, the new mechanism indicates that cost-effective applications may be developed based on this new knowledge.
"As we began to realize our results, it took us quite some time to understand what we were observing and why, but the result is exciting and motivates us to dig deeper into how surface phase transitions can change the chemical environment," says Erik S. Thomson, senior lecturer at the University of Gothenburg and one of the authors behind the study.
More information: Xiangrui Kong et al, A surface-promoted redox reaction occurs spontaneously on solvating inorganic aerosol surfaces, Science (2021). DOI: 10.1126/science.abc5311
Journal information: Science
Provided by University of Gothenburg