Frizzleds are dynamic, molecular machines
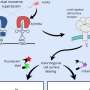
Maria Kowalski-Jahn and Hannes Schihada, two postdocs in the Schulte laboratory, have used a novel technology of fluorescently labeling receptors with a minimally invasive technique and detecting structural rearrangements in a receptor molecule in living cells. These experiments pinpointed how FZDs respond to WNT stimulation by conformational changes in the extracellular domain of the receptor. In contrast to what was previously surmised, but in line with several previous publications by the Schulte laboratory, these findings underline that WNT stimulation elicits conformational rearrangements in their receptors. These new insights into FZD dynamics present the basis for a continued mechanism-based drug discovery process to target FZDs for therapy.
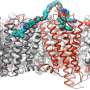
Cell surface receptors are essential to transmit information from the outside of the cell to the inside to accomplish a physiological adaptation in response to the perceived signal. This basic concept of cellular communication can be exploited by therapeutic intervention using drugs that either mimic the bodily molecule activating the receptor (agonists) or interfere with binding of the bodily molecule as so-called antagonists. Irrespectively, the dynamic process of receptor activation can only be targeted if it is understood in its basic details. The Schulte laboratory works on Class F G protein-coupled receptors including Frizzleds, which are seen as attractive drug targets for example for cancer therapy. So far, however, the molecular underpinnings of ligand (WNT)-induced FZD activation remains obscure.
This study is published in Science Advances.
More information: Maria Kowalski-Jahn et al, Frizzled BRET sensors based on bioorthogonal labeling of unnatural amino acids reveal WNT-induced dynamics of the cysteine-rich domain, Science Advances (2021). DOI: 10.1126/sciadv.abj7917
Journal information: Science Advances
Provided by Karolinska Institutet