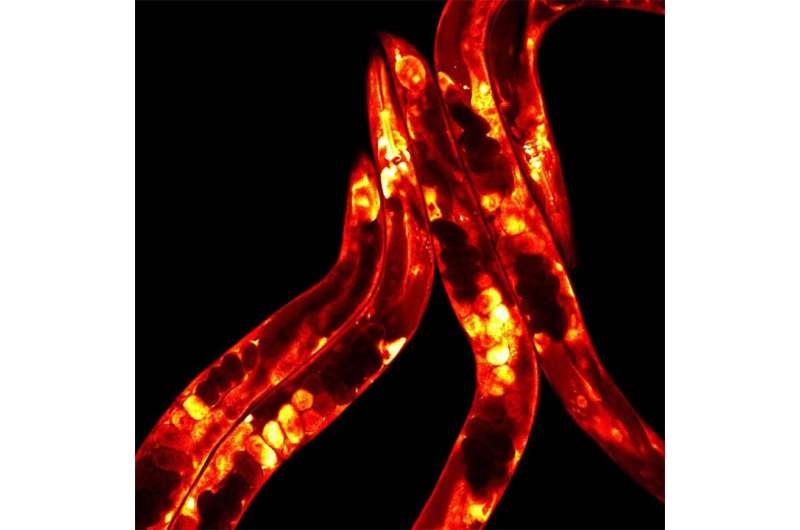
C. elegans worms labelled with a fluorescent protein and imaged using a confocal microscope. Credit: Babraham Institute
How our bodies sense and respond to environmental changes are fundamental biological questions. In particular, understanding how organisms sense and cope with warming temperatures is key for the survival of species and it will become an even more important area of research given the raising trend in the earth's temperature. In research recently published in PLOS Biology, researchers from the Casanueva group used the tiny nematode worm C. elegans to show for the first time that although ectotherms cannot internally regulate their own body temperature as mammals do, they are able to centrally control their response to heat.
Rising temperatures are problematic for cell membranes, which are mostly made of fat molecules called lipids. Lipids are extremely sensitive to temperature change and must be regulated for cell membranes to be able to maintain their function. Multicellular organisms face an additional challenge: membrane lipids are only produced and kept in specialized fat storage tissues. The question is how can distant tissues get the right type of lipids to adapt their membranes in response to temperature change?
Endotherms, such as mammals and birds, maintain their body temperature by using peripheral sensors to relay information to the brain, where specific neurons function as a type of thermostat that sets and maintains a healthy body temperature. By contrast, ectotherms, such as reptiles, fish, and invertebrates, cannot control their own body temperature. For invertebrates like C. elegans, it was thought that this process was controlled independently by individual cells rather than coordinated on an organism wide level.
Dr. Olivia Casanueva and her team have shown that in C. elegans a group of neurons in worms act by registering external temperature and coordinating fat remodeling. This process is a result of a conserved response that exists in all animals called the heat shock response. When exposed to heat stress, cells activate the Heat Shock Response pathway during which the signaling molecule Heat Shock Factor 1 (HSF-1) rapidly induces the production of heat shock proteins (HSPs), which are themselves involved in repairing heat-damaged proteins. HSF-1 is also recognized as a pro-longevity factor because it clears aggregates and misfolded proteins that accumulate in old cells.
In this study, Dr. Casanueva found that in some sensory neurons, HSF-1 responds to slight changes in temperature and sends signals through a multistep response to the gut, where fats are made. The team took advantage of the transparency of the worm's cuticle to visualize fat metabolism in individual worms. They found that HSF-1 is necessary and sufficient to coordinate a complex neuro-hormonal response between the neurones detecting temperature change and fat producing gut cells.
"This is the first study to show that ectotherms utilize an ancient heat sensing cellular response as a thermostat to centrally coordinate complex adaptive responses to warming temperatures." said Dr. Casanueva, "This response ensures that the right type of fats are made for membrane remodeling across the organism's tissues and allows worms to survive warming temperatures."
By better understanding how invertebrates regulate fat composition in the body, future research will be able to identify if a similar role has been co-opted in mammals, which may be important in the context of aging and obesity in humans.
More information: Laetitia Chauve et al, Neuronal HSF-1 coordinates the propagation of fat desaturation across tissues to enable adaptation to high temperatures in C. elegans, PLOS Biology (2021). DOI: 10.1371/journal.pbio.3001431
Journal information: PLoS Biology
Provided by Babraham Institute