Creating and studying radioactive molecules advances nuclear structure and fundamental symmetry studies
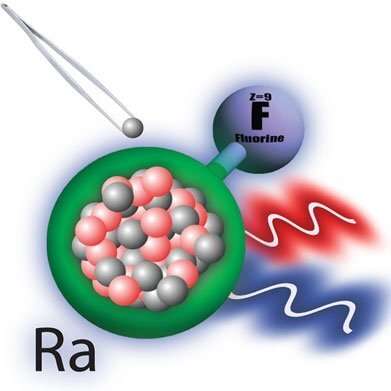
An international team performed the world's first measurement of how the size of the radium nucleus modifies the structure of molecules containing different radium isotopes. The research used a combination of lasers and ion traps at the Isotope mass Separator On-Line (ISOLDE) Radioactive Ion Beam Facility at CERN. The team studied the quantum structure of radium monofluoride (RaF) molecules. Quantum structure dictates the energy levels and how these levels change under different conditions. Scientists predict that RaF molecules are useful for studying the violation of certain fundamental symmetries found in nature. The team measured the changes in electronic energy levels when one of the radium nuclei was replaced with a different isotope. This demonstrates the extreme sensitivity of these molecules to the interaction of electrons and nuclei at short distances.
The ability to precisely measure energy levels and modify the number of neutrons in the nuclei of the molecules opens new directions for research. The Big Bang should have created equal amounts of matter and antimatter. Violations of fundamental symmetries can explain why there is more matter than antimatter in our universe. Radioactive molecules containing isotopes of heavy elements like radium are ideal for studying the violation of these fundamental symmetries. Scientists also believe that their experimental developments could be applied to study other radioactive molecules that are created in supernovae and other stellar explosions. But scientists' limited observational tools have prevented their identification in space. Thus, laboratory studies of radioactive molecules will help guide future astrophysical observations.
Radioactive molecules promise exciting new opportunities at the frontiers of fundamental physics and chemistry. However, they are very rare in nature, and some do not occur in nature at all. This means they must be created artificially at specialized facilities. Moreover, their lifetimes can be as short as a few days or a fraction of a second, so studying them requires extremely sensitive experimental techniques. The Facility for Rare Isotope Beams (FRIB), a Department of Energy (DOE) user facility that will begin operations in 2022 will provide unique access to molecules containing isotopes of the heaviest elements. Future developments of the current techniques at this facility will offer a new platform for discoveries in fundamental physics. This will advance understanding of the fundamental symmetries of nature, as well as an understanding of the chemistry and nuclear structure of the heavy elements.
More information: S. M. Udrescu et al, Isotope Shifts of Radium Monofluoride Molecules, Physical Review Letters (2021). DOI: 10.1103/PhysRevLett.127.033001
Journal information: Physical Review Letters
Provided by US Department of Energy