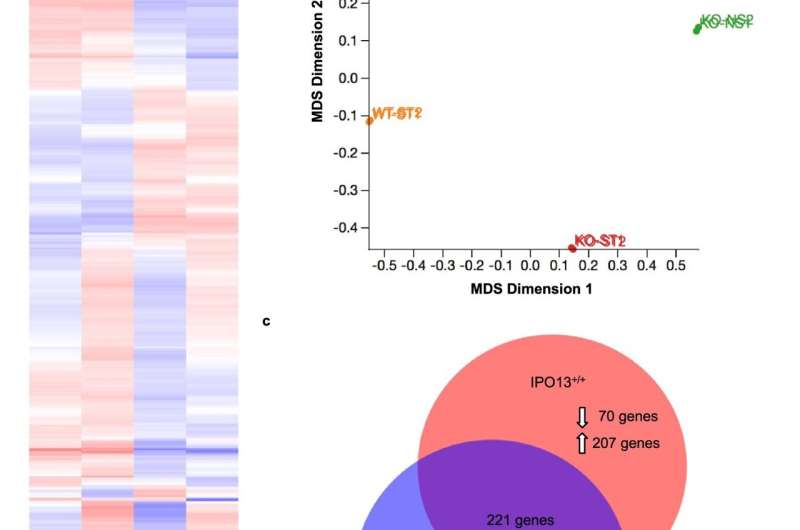
Fig. 1: The IPO13-dependent transcriptome in oxidative stress. a Heat-map of transcript data for IPO13+/+ and IPO13−/− mESCs with or without treatment with 125 μM hydrogen peroxide (H2O2) for 1 h followed by 2 h recovery (ST; stress treated). Log2-fold differences in expression across the four data sets (FDR cut-off = 0.05) are shown for 2352 gene models (horizontal axis). b Multi-dimensional scaling (MDS) plot summarizing gene expression profiles of IPO13+/+ (“WT”) and IPO13−/− (“KO”) ESCs for control (NS; no stress) and oxidative stress-treated (ST) conditions. c Venn diagram for the differentially expressed genes in response to stress in IPO13+/+ and IPO13−/− ESCs (498 and 521 genes, respectively). d MA plot showing differentially expressed genes with a log2-fold change increase or decrease in expression (FDR cut-off = 0.05) in response to oxidative stress compared to untreated ESCs. Log fold change (logFC) values are plotted against log expression values (standardized read counts). Genes highlighted in yellow were selected for validation. e–f IPO13+/+ and IPO13−/− ESCs were treated with 125 µM H2O2 as indicated for 1 h and then stained for 30 min with 5 µM CellROX Green either immediately, or after a 2 h recovery period, and then analysed by flow cytometry. Quantification of the median fluorescence intensity relative to the untreated (UT) sample are shown representing the mean ± SEM (n = 3 independent experiments); p values (two-tailed student’s t-test) top to bottom left to right: p = 0.0617, p = 0.0479, p = 0.2167, and p = 0.0136. Source data are provided as a Source Data file. Credit: DOI: 10.1038/s41467-021-26125-x
Scientists at Monash University's Biomedicine Discovery Institute (BDI) have discovered a new role for a known protein in oxidative stress, which is linked to diseases such as Alzheimer's and Parkinson's, diabetes, cardiovascular diseases, viral infection and cancer.
When cells are stressed they get damaged; the cell can then either repair itself or if the damage is too great it can die. The BDI researchers looked at oxidative stress, a form of chemical stress, and found that the Importin-13 protein promotes cellular death pathways in response to stress.
The study, led by Professor David Jans and conducted by post-doctoral researcher Dr. Kasia Gajewska, identified a new pathway regulated by Importin-13, which belongs to a superfamily of transporters in cells that move proteins, RNAs, and protein-RNA complexes in and out of the control center of the cell and the nucleus, helping to regulate stress response pathways impacting cell survival.
The findings are now published in Nature Communications.
"We were able to identify that quite a lot of genes that respond to stress are dependent on Importin-13," Dr. Gajewska said.
"No one has really worked out that Importin-13 is important in stress—in fact, it may be one of the key things in responding to stress," Professor Jans said.
"Our findings have relevance to other forms of stress such as heat stress relevant to climate change," Dr. Gajewska said. "Understanding the molecular basis of the impact of stress is more important than ever before."
Importin-13 is conserved widely in evolution, meaning that the pathway potentially relates to many different organisms, including plants.
Although a basic science study, its authors are flagging Importin-13 as a potential target for drug development.
"Kasia has demonstrated that Importin-13 is of great interest as a target for an inhibitor," Professor Jans said. "Inhibitors against these sorts of transporters have already been identified—we have worked on several for other transporters.
"This was a fantastic piece of work by Kasia," he said. "It has great implications."
Dr. Gajewska, who works in Professor Jans' Nuclear Signalling lab, was guided in bioinformatics by co-author Dr. Mirana Ramialison from the Australian Regenerative Medicine Institute at Monash to conduct the bioinformatics and analyze the transcriptomic data generated, which used a knockout cell line. She was co-supervised by Professor Jans and Dr. Kylie Wagstaff.
The study builds on previous work into Importin-13 by Professor Jans and Dr. Kylie Wagstaff that spans a decade.
Dr. Gajewska is currently working on Importin-13's critical role in neuronal differentiation. She is keen to pursue studies into the potential role of the protein in neurodegenerative diseases since oxidative stress is thought to contribute to neurological cell death and dysfunction.
The paper in Nature Communications is titled "Nuclear transporter Importin-13 plays a key role in the oxidative stress transcriptional response."
More information: K. A. Gajewska et al, Nuclear transporter Importin-13 plays a key role in the oxidative stress transcriptional response, Nature Communications (2021). DOI: 10.1038/s41467-021-26125-x
Journal information: Nature Communications
Provided by Monash University