Researchers at Graz University of Technology have developed a new, significantly cheaper production method for germanium-based photoinitiators. This opens up further fields of application beyond the dental sector. Credit: Frankl - TU Graz
Photoinitiators ensure that liquid plastic—for example for dental fillings—hardens quickly by means of light. Thanks to a new synthesis method developed by TU Graz, these initiators can be produced cheaply, something which will open up further doors for the technology.
Anyone who has ever been in the dentist's chair with a hole in their tooth is probably familiar with the procedure. After the hole in the tooth is drilled, a filling made of liquid plastic is inserted. This is then modeled in the mouth and hardened (cured) by UV light. This is made possible by so-called photoinitiators. These are chemical compounds that are added to the filling paste. They decompose when exposed to light and form radicals that cause this paste to harden.
For some years now, germanium-based photoinitiators have been used for this purpose. The advantage of these is that they absorb light with longer wavelength and therefore do not require UV light, which is hazardous to health, for curing. This non-toxic photoinitiator has already established itself in the dental field, although it is expensive to produce. The production costs of one kilogram of this initiator are currently in the order of magnitude of a new small car. "Given the small quantities needed for dental fillings, the price of the photoinitiator is hardly a factor in the dental industry. For other applications, however, the expensive production was a stumbling block—until now," explains chemist Michael Haas from Graz University of Technology (TU Graz).
New, simple method of synthesis
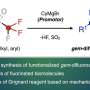
Together with his team at the Institute of Inorganic Chemistry, Haas has developed a completely new method of synthesis for germanium-based photoinitiators. In contrast to conventional synthesis, this production method not only does not require sulfur ("a smell you don't necessarily want in your mouth"), but is also significantly simpler, more efficient and cheaper. We have succeeded in establishing an alternative approach to this class of compounds that is single-step and makes isolating the product absolutely simple." In the process, several silicon-based protective groups are cleaved off simultaneously. The desired compound is then isolated by simple crystallization. This opens up further biomedical applications for this class of photoinitiators, for example in the production of contact lenses, prostheses, novel implants and artificial human tissue.
The researchers have now translated this alternative approach into application with the project partner Ivoclar Vivadent AG. The dental company already had a toxicologically safe germanium-based photoinitiator (Ivocerin) in its product portfolio. However, this also has serious disadvantages in production, as Haas explains: "In the case of Ivocerin, the synthesis is complex and multi-step process, and the removal of the reaction partners is also expensive and leads to enormous yield losses." The foreseeable market launch of the new initiator will make dental fillings significantly cheaper in the future.
Michael Haas also sees potential for other biomedical applications, such as contact lenses. For most of these applications, toxicologically questionable photoinitiators have been used up to now (e. g. phosphorus-based initiators). The germanium-based initiators, which are harmless to health, have so far been too expensive for these applications. The production of novel implants, prostheses or artificial human tissue are also possible areas of application for the newly synthesized initiator.
"It becomes interesting wherever the use of non-toxic materials is of central importance," says Haas. At around 12 years old, research on photoinitiators is a relatively young field. Michael Haas and his research group have already successfully been granted two independent patents in the field of germanium-based photoinitiators in the past four years. "Since radical photoinitiators are used in many industrial processes, the absolute relevance of our results cannot yet be assessed," says Haas.
For all its application orientation, Michael Haas' working group is also reaping a rich harvest in basic research. In recent years, they have published more than 15 papers in recognized scientific journals in this field alone. Haas, together with his doctoral student Manfred Drusgala and other colleagues, has recently published new results in the scientific journal Angewandte Chemie. In it, the researchers describe a new method for the targeted synthesis of so-called bisenolates, a special class of compounds from enolate chemistry. This class of compounds is characterized by the possibility of a double reaction at the central and active germanium atom—i.e. two reactions can be carried out simultaneously. This allows the introduction of new functionalities, making this new class of compounds of great interest for further research in the field of photoinitiators.
"This is also a milestone for the entire field of organometallic chemistry," says Haas. He and his team are currently developing completely new types of water-soluble photoinitiators based on these molecules, something which represents previously untrodden ground in this field of research.
More information: Manfred Drusgala et al, Isolable Geminal Bisgermenolates: A New Synthon in Organometallic Chemistry, Angewandte Chemie International Edition (2021). DOI: 10.1002/anie.202111636
Journal information: Angewandte Chemie International Edition , Angewandte Chemie
Provided by Graz University of Technology