Graphical abstract. Credit: DOI: 10.1016/j.stem.2021.08.010
In a study published in Cell Stem Cell, the research group led by Dr. Zhou Bo from the Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences reported the transition of skeletal progenitor during postnatal bone growth.
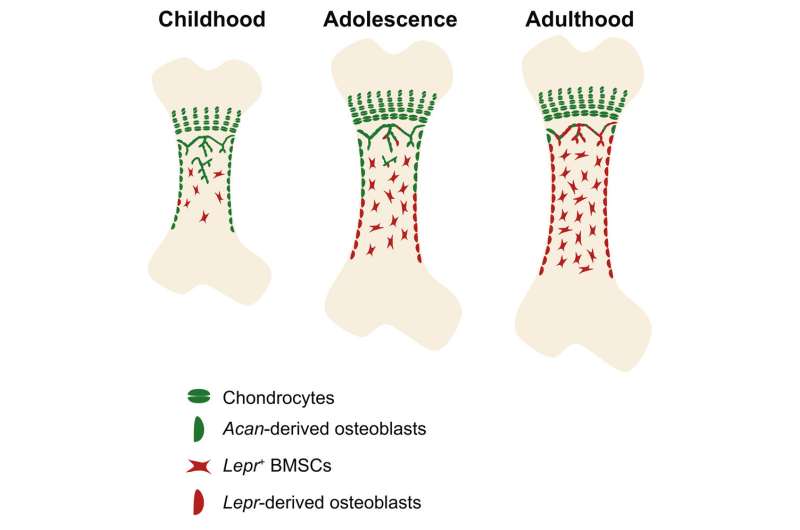
Postnatal bone growth and regeneration rely on the osteoblasts formed by skeletal progenitor. Multiple distinct cell types have been identified as skeletal progenitors, including LepR+ bone marrow stromal cells (BMSCs) and postnatal Acan+ chondrocytes.
LepR+ BMSCs resided in the perivascular region in the bone marrow, giving rise to osteoblasts and adipocytes under physiological condition, while postnatal Acan+ chondrocytes occupied in the growth plate, forming osteoblasts and also BMSCs. However, the functional heterogeneity and hierarchical relationship between these two distinct skeletal progenitors remain unknown.
In this study, scientists took advantage of lineage tracing tools to fate-mapping the chondrocytes and LepR+ BMSCs at different stage. They found that bone formation was sequentially controlled by perinatal chondrocytes (before adolescence) and adult LepR+ cells (after adolescence).
To investigate the hierarchical relationship between chondrocytes and LepR+ BMSCs, scientists developed dual-recombinase system to simultaneously trace the fate of chondrocytes and LepR+ BMSCs. The data showed that the majority of LepR+ cells and their lineages were descendant from fetal chondrocytes, and that the Lepr-derived osteoblasts and Acan-derived osteoblasts displayed a gradient distribution pattern.
Besides, scientists found that conditional knock out Runx2, the master regulator for osteogenic differentiation, in perinatal chondrocytes and adult LepR+ cells impaired bone growth and thickening, respectively, and that under exercise stimuli, compared with adult LepR+ cells, the perinatal chondrocytes were more inclined to differentiate into osteoblasts.
This study emphasized the temporally and spatially transition of skeletal progenitors at different stage of bone development, uncovered the hierarchical relationship in distinct skeletal progenitors in development, and explained, in a stem cell perspective, how mammalian limb bones transit from rapid longitudinal growth to much slower appositional maintenance after adolescence.
More information: Hui Sophie Shu et al, Tracing the skeletal progenitor transition during postnatal bone formation, Cell Stem Cell (2021). DOI: 10.1016/j.stem.2021.08.010
Journal information: Cell Stem Cell
Provided by Chinese Academy of Sciences