How to prevent cathodic corrosion of metal electrodes in electroorganic synthesis
Sustainability is an important issue in business and industry these days. Many companies recognize the need to find the best possible climate-neutral solutions for manufacturing their products and reduce their output of pollutants. This means they are looking for manufacturing options that do not require the use of fossil raw materials. Great potential in this respect is seen in electrosynthesis, a process that involves the transformation of chemical substances in an electrolysis cell using electrical energy.
A team of researchers led by Professor Siegfried Waldvogel, spokesperson of the SusInnoScience Top-Level Research Area at Johannes Gutenberg University Mainz (JGU), has already demonstrated, for example, that it is possible to use this technique to extract the flavor compound vanillin from wood waste. One particularly promising application of electrosynthesis would be its use for the production of plastics precursors. Electrosynthesis would not only be more efficient than the conventional techniques but also would not entail the consumption of fossil resources. However, there is a significant and as yet largely overlooked snag: During electrosynthesis, a process known as cathodic corrosion occurs. Waldvogel's team decided to explore this issue more deeply by first undertaking a review of literature on the subject. The results of that research have been published recently in Chemical Reviews.
The research team assessed articles dealing with cathodic corrosion that have appeared over the past 130 years, including some 30 papers that they themselves produced. "Our team and a Chinese group are the only ones with the necessary expertise to carry out such a literature review," emphasized Waldvogel.
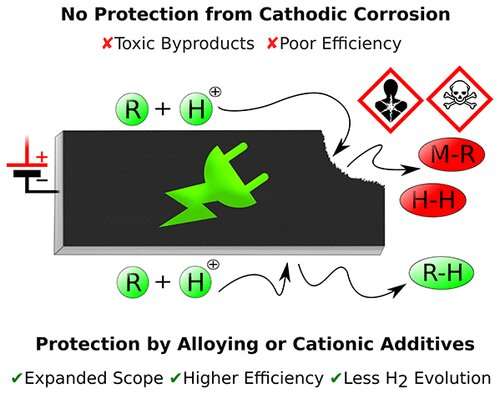
According to Waldvogel, scientists have been aware of the problem of cathodic corrosion for more than 200 years, but a means of preventing it has still not been found. While the oxidation of the positive electrode, the anode, during electrolysis has been thoroughly investigated, there are still many unanswered questions concerning the reduction that happens at the negative electrode, the cathode. "It is necessary to use materials for the electrodes that have a high overpotential with regard to hydrogen, so for that reason toxic heavy metals, such as lead and tin, are employed," said Waldvogel. "However, the cathode gradually dissolves—or corrodes—and releases these poisonous metals." This can result in contamination of the synthesized chemicals, which is, of course, an undesirable effect. "If we were able to prevent this corrosion, we would have removed one of the major stumbling blocks on the road to electrification of production processes," he added. The chemist is currently working on two projects designed to find a solution to the problem. The project called "Strategies to Overcome Contemporary Limitations of Reductive Electrosynthetic Conversions in Aqueous Media" has just been initiated this month. It is being financed by the German Research Foundation (DFG) and the US National Science Foundation to the tune of roughly EUR 1 million.
The focus here is on a practical application. Working in collaboration with a team at Iowa State University, the objective is to develop a method of generating precursors for plastics from agricultural waste—and these products are to be synthesized at the cathode. "Assuming we are successful, we will in future be able to use waste to make chemical intermediates, resulting in sustainable value enhancement," stated Waldvogel. According to the team at Mainz University, they will be mainly considering the various ways in which electrodes can be coated by salts while their American counterparts will be focusing on the use of alloys with which it is hoped that cathodic corrosion can be inhibited.
Since early 2021, researchers of the two JGU Top-Level Research Areas SusInnoScience and M3ODEL have been together working on the ECHELON project, for which the Carl Zeiss Foundation is providing some EUR 2 million in funding. "The aim is to gain a better understanding of the underlying theory of the processes that occur during electrolysis. For this purpose, we are combining aspects of the two important fields of quantum chemistry and multi-scale modeling," said Waldvogel. "Quantum chemistry allows us to calculate the chemical reactions at the cathode, while multi-scale modeling enables us to theoretically map the movement and concentration of the ions in the fluid surrounding the cathode," he concluded.
More information: Tom Wirtanen et al, Cathodic Corrosion of Metal Electrodes—How to Prevent It in Electroorganic Synthesis, Chemical Reviews (2021). DOI: 10.1021/acs.chemrev.1c00148
Journal information: Chemical Reviews
Provided by Universitaet Mainz