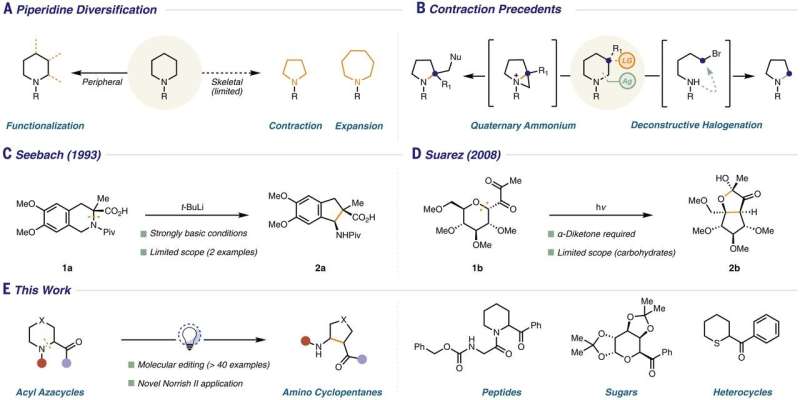
Approaches to piperidine diversification. (A) Peripheral functionalization and skeletal remodeling. (B) Selected examples of ring contractions on piperidine frameworks. (C) Seminal report of Seebach and co-workers’ unusual THIQ ring contraction (20). (D) Contraction of carbohydrates reported by Suárez and co-workers (21). (E) Norrish type II approach to piperidine skeletal framework modification (this work). Credit: Science (2021). DOI: 10.1126/science.abi7183
A team of chemists at the University of California, Berkeley, working with a group at Merck & Co. Inc. has developed a reaction that can be used to remove a single sulfur, nitrogen or oxygen atom from a six-membered ring using only a blue light. In their paper published in the journal Science the group describes their reaction and possible uses for it in various applications.
In recent years, chemists have found ways to manipulate the carbon rings that are used to create a host of pharmaceutical and agrochemical products, but most are complex and difficult to conduct. They also typically involve using a lot of energy. In this new effort, the researchers have found a way to break the C–S, C–O and C–N bonds in saturated heterocycle rings to remove an atom, using only a blue light—afterward, the ring recloses, with one less atom.
To carry out a reaction, the researchers first expose an aromatic ketone group (which is attached to a heterocycle) to a blue LED light. This sets off a Norrish reaction, wherein a carbon-heteroatom bond is cleaved, resulting in the opening of the ring and the ejection of an atom. This works because it results in the creation of a core radical. The second part of the reaction involves closure of the ring, which, the team notes, involves a procedure similar to a Mannich reaction. They note that for the reaction to work, the light wavelength had to match exactly with the carbonyl in the starting material. They also note that no atoms were removed from the molecule, nor were any added—the structure was simply rearranged in a way that left a five-membered ring.
The researchers note that despite its simplicity, the reaction is a game-changer for manipulating the building blocks of medicinal chemistry. They note also that the reaction could likely be used in combination with other reactions—offering a 1-2 punch sort of reaction to make more than one modification at a time. They demonstrated the effectiveness of their reaction by using it to edit multiple well-known drugs, such as mefloquine and rimiterol. They also noted that their new goal is to find a way to add an aromatic ketone to a saturated heterocycle.
More information: Justin Jurczyk et al, Photomediated ring contraction of saturated heterocycles, Science (2021). DOI: 10.1126/science.abi7183
Journal information: Science
© 2021 Science X Network