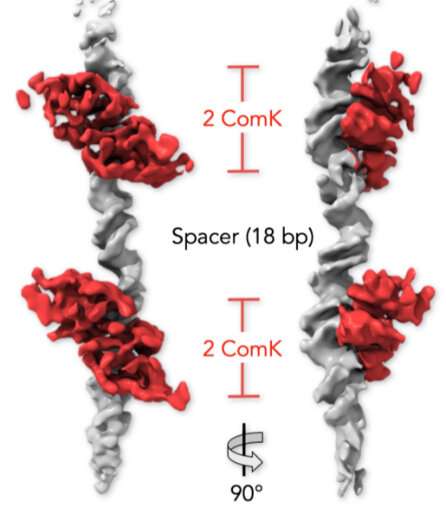
A 3D reconstruction from single particles of bacterial DNA (gray) and ComK proteins (red), imaged by cryogenic electron microscopy, viewed from the front (left) and at a 900 rotation. ComK molecules bound to two sites communicate through the DNA segment between them Credit: Weizmann Institute of Science
Proteins can communicate through DNA, conducting a long-distance dialog that serves as a kind of genetic "switch," according to Weizmann Institute of Science researchers. They found that the binding of proteins to one site of a DNA molecule can physically affect another binding site at a distant location, and that this "peer effect" activates certain genes. This effect had previously been observed in artificial systems, but the Weizmann study is the first to show it takes place in the DNA of living organisms.
A team headed by Dr. Hagen Hofmann of the Chemical and Structural Biology Department made this discovery while studying a peculiar phenomenon in the soil bacteria Bacillus subtilis. A small minority of these bacteria demonstrate a unique skill: an ability to enrich their genomes by taking up bacterial gene segments scattered in the soil around them. This ability depends on a protein called ComK, a transcription factor, which binds to the DNA to activate the genes that make the scavenging possible. However, it was unknown how exactly this activation works.
Staff Scientist Dr. Gabriel Rosenblum led this study, in which the researchers explored the bacterial DNA using advanced biophysical tools—single-molecule FRET and cryogenic electron microscopy. In particular, they focused on the two sites on the DNA molecule to which ComK proteins bind.
They found that when two ComK molecules bind to one of the sites, it sets off a signal that facilitates the binding of two additional ComK molecules at the second site. The signal can travel between the sites because physical changes triggered by the original proteins' binding create tension that is transmitted along the DNA, something like twisting a rope from one end. Once all four molecules are bound to the DNA, a threshold is passed, switching on the bacterium's gene scavenging ability.
"We were surprised to discover that DNA, in addition to containing the genetic code, acts like a communication cable, transmitting information over a relatively long distance from one protein binding site to another," Rosenblum says.
By manipulating the bacterial DNA and monitoring the effects of these manipulations, the scientists clarified the details of the long-distance communication within the DNA. They found that for communication—or cooperation—between two sites to occur, these sites must be located at a particular distance from one another, and they must face the same direction on the DNA helix. Any deviation from these two conditions—for example, increasing the distance—weakened the communication. The sequence of genetic letters running between the two sites was found to have little effect on this communication, whereas a break in the DNA interrupted it completely, providing further evidence that this communication occurs through a physical connection.
Knowing these details may help design molecular switches of desired strengths for a variety of applications. The latter may include genetically engineering bacteria to clean up environmental pollution or synthesizing enzymes to be used as drugs.
"Long-distance communication within a DNA molecule is a new type of regulatory mechanism—one that opens up previously unavailable methods for designing the genetic circuits of the future," Hofmann says.
The study was published in Nature Communications.
More information: Gabriel Rosenblum et al, Allostery through DNA drives phenotype switching, Nature Communications (2021). DOI: 10.1038/s41467-021-23148-2
Journal information: Nature Communications
Provided by Weizmann Institute of Science