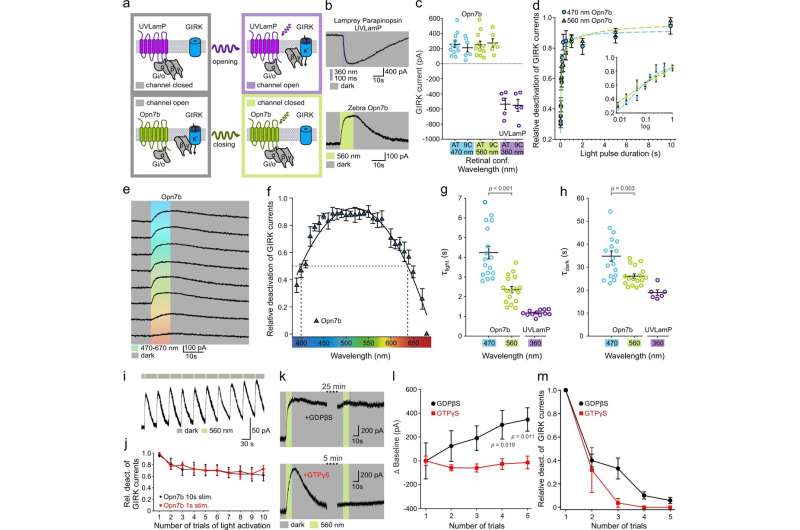
Fig. 1: Opn7b constitutively activates the Gi/o pathway in HEK293 cells. a Schematic representation of Gi/o mediated (de-)activation of G protein-coupled inwardly rectifying potassium (GIRK) channels illustrating the reverted functionality of Opn7b (bottom) in contrast to classical G protein-coupled receptor (GPCR)-type opsins (top); i.e., lamprey parapinopsin (UVLamP). Typical GPCR-type opsins convert from their dark-adapted, stable resting or inactive state to their G protein-activating state upon photon absorption (monostable) or interconvert between both states in a light-dependent manner (multistable). In contrast to both groups, the monostable Gi/o-coupled Opn7b exhibits a dark-adapted, stable G protein-activating state and is inactivated upon photon absorption. b Patch-clamp recording example traces of GIRK currents. The classical Gi/o-coupled GPCR-type opsin UVLamP induces G protein-mediated opening of co-expressed GIRK channels (inward K+ current under given experimental conditions) upon light stimulation. In contrast, under the same experimental conditions, in darkness, Gi/o-coupled Opn7b leads to sustained G protein-mediated opening of co-expressed GIRK channels that are closed upon light stimulation. c Comparison of light (de-)activated GIRK current amplitude for UVLamP (n = 5 cells) and Opn7b (all-trans n = 11 cells, 9-cis n = 6 cells), using all-trans (AT) or 9-cis (9 C) retinal and light of the indicated wavelengths. Mean values (±SEM) and single-cell data (circles) are shown. d Light pulse duration dependence of Opn7b inactivation using light of the indicated wavelengths. Mean values (± SEM) are shown (n = 4 cells per group). e Patch-clamp recording example traces of GIRK currents for Opn7b at different wavelengths. f Action spectrum depicting the wavelength dependence of Opn7b inactivation. Mean values (±SEM) are shown (n = 5 cells). g Comparison of light-induced GIRK current (de-)activation time constants (τlight) for Opn7b (n = 17 cells) and UVLamP (n = 12 cells) at the indicated wavelengths. Mean values (±SEM) and single-cell data (circles) are shown (two-sided Mann–Whitney-U-test). h Comparison of darkness-induced post-stimulus GIRK current (de-)activation time constants (τdark) for Opn7b (n = 17 cells) and UVLamP (n = 6 cells) at the indicated wavelengths. Mean values (±SEM) and single-cell data (circles) are shown (two-sided Mann–Whitney-U-test). i Patch-clamp recording example traces of GIRK currents for repetitive activation of Opn7b. j Relative GIRK current amplitude for repetitive activation of Opn7b. Mean values (±SEM) are shown (10 s stim n = 5 cells, 1 s stim n = 6 cells). k Patch-clamp recording example traces of GIRK currents for Opn7b under pipette-mediated (intracellular) application of guanosine diphosphate beta S (GDPβS, top) or guanosine triphosphate gamma S (GTPγS, bottom) at different time points after establishment of whole-cell configuration. l Comparison of GIRK current baseline for repetitive activation of Opn7b under application of GDPβS (black) or GTPγS (red) at different time points after establishment of whole-cell configuration. Mean values (±SEM) are shown (n = 5 cells per group; two-way repeated measure ANOVA followed by Bonferroni test, F(1,8) = 4.881, p = 0.058). m Relative GIRK current amplitude for repetitive activation of Opn7b under application of GDPβS (black) or GTPγS (red) at different time points after establishment of whole-cell configuration. Mean values (±SEM) are shown (n = 5 cells per group). Source data are provided as a Source Data file. Credit: DOI: 10.1038/s41467-021-24718-0
A new optogenetic tool, a protein that can be controlled by light, has been characterized by researchers at Ruhr-Universität Bochum (RUB). They used an opsin—a protein that occurs in the brain and eyes—from zebrafish and introduced it into the brain of mice. Unlike other optogenetic tools, this opsin is not switched on but rather switched off by light. Experiments also showed that the tool could be suitable for investigating changes in the brain that are responsible for the development of epilepsy.
The teams led by Professor Melanie Mark from the Behavioral Neurobiology Research Group and Professor Stefan Herlitze from the Department of General Zoology and Neurobiology describe the experiments and results in the journal Nature Communications, published online on 23 July 2021.
Role assumed in various conditions
The opsin Opn7b is a G protein-coupled receptor which is found in zebrafish. Unlike many other light-activated G protein-coupled receptors, it can be activated without a light stimulus and is thus permanently active; researchers call this constitutively active. Normally, activation of G protein-coupled receptors leads to an opening of certain ion channels and thus to the influx of ions into the cell as well as to further signaling processes in the cell. In the case of Opn7b, light deactivates this permanently active signaling chain.
Little research has so far been conducted on G protein-coupled receptors that are activated without stimulation, although it is presumed that they play a role in various neuropsychiatric conditions and night blindness. They also appear to be involved in the development of virally induced cancers.
Receptor characterized more precisely
Dr. Raziye Karapinar, Dr. Ida Siveke and Dr. Dennis Eickelbeck characterized the function of Opn7b in detail and, to their surprise, identified that the receptor is deactivated by light. In contrast, conventional optogenetic tools are switched on by light.
The researchers consider Opn7b well-suited to gain further insights into the function of G protein-coupled receptors that are constitutively active—and obtain new knowledge of their role in the development of diseases in which the receptors can be examined in a time-controlled manner in specific cell types.
Epileptic seizures
The Bochum researchers Dr. Jan Claudius Schwitalla and Johanna Pakusch changed certain cells in the cerebral cortex of mice in such a way that they produced Opn7b. If they deactivated the receptor with light, it triggered epileptiform activity in the animals, which could be specifically controlled with light and interrupted with the help of other light-controlled proteins. The researchers hope that it will be possible to use this optogenetic tool to understand more precisely both the underlying mechanisms and the timescales in the development of epileptic seizures.
More information: Raziye Karapinar et al, Reverse optogenetics of G protein signaling by zebrafish non-visual opsin Opn7b for synchronization of neuronal networks, Nature Communications (2021). DOI: 10.1038/s41467-021-24718-0
Journal information: Nature Communications
Provided by Ruhr-Universitaet-Bochum