Ball and stick model of methane. Credit: Ben Mills/Public Domain
Biomethane (CH4) can be used as feedstock for modern chemical industry or burned directly as a fuel.
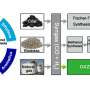
Currently, CH4 is mainly produced via a multi-step process in which biomass is first gasified into biogas, followed by the methanation of the latter. This method requires high temperature and pressure and shows low selectivity for chemical or biological processes.
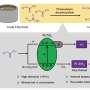
Recently, a research group led by Prof. Wang Feng from the Dalian Institute of Chemical Physics of the Chinese Academy of Sciences, in collaboration with Prof. Wang Min's group from Dalian University of Technology, proposed interfacial oxygen-vacancy (Vo)-mediated catalysis over Ru/TiO2 for the direct methanation of lignocellulosic biomass at temperatures below 200°C and with a selectivity above 95%.
The results were published in Joule on July 27.
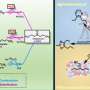
"We proposed the Vo-mediated catalysis process to couple the oxidation of biomass into CO2 with the hydrogenation of CO2 into CH4, leading to the direct methanation of biomass under mild conditions," said Prof. WANG Min.
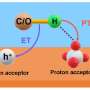
The researchers found that the biomass substrate molecule was oxidized by the lattice oxygen of Ru/P25 into CO2, and Vo formed on Ru/P25. Subsequently, the dissociated oxygen atoms derived from CO2 could restore the Vo during the CO2 hydrogenation process.
Moreover, they found that the Vo-mediated catalysis process could stably catalyze the production of CH4 from aqueous glycerol at temperatures as low as 120 °C and with a selectivity above 99%.
"This direct methanation process is simpler and more efficient than the traditional two-step process of biogas production and methanation," said Prof. Wang Feng. "It opens up a new route for the utilization of biomass resources."
More information: Hongru Zhou et al, Oxygen-vacancy-mediated catalytic methanation of lignocellulose at temperatures below 200°C, Joule (2021). DOI: 10.1016/j.joule.2021.07.001
Journal information: Joule
Provided by Chinese Academy of Sciences