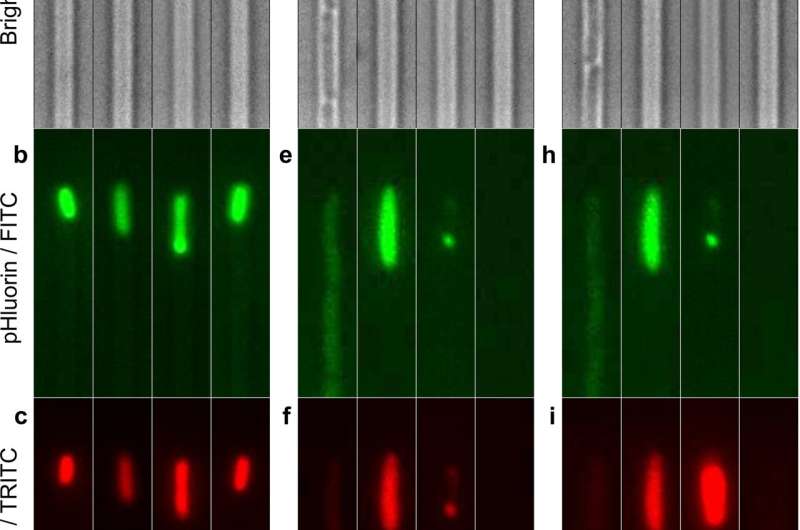
Fluorescence measurement of intracellular pH in individual persister, VBNC, or susceptible E. coli cells before and after ampicillin treatment. (a to c) Bright-field, FITC, and TRITC fluorescence images of four representative bacteria injected in the microfluidic mother machine from a 2 μl aliquot that was prepared as follows. A 200 ml 17 h stationary phase E. coli BW25113 culture, with a typical OD595 of 5, was spun down and resuspended in filtered medium (from the overnight culture) to be adjusted to an OD of 75. 2 μl were withdrawn from this sample and injected into the mother machine. (d to f) Corresponding images of the same bacteria after 3 h of incubation in ampicillin at a concentration of 25× the MIC in an M9-LB (90:10, vol/vol) solution and a successive 21 h of incubation with fresh nutrients. (g to i) Corresponding images of the same bacteria after a 20 min incubation in propidium iodide (PI) to distinguish between susceptible bacteria (second channel from the right), in which PI addition increases the mCherry red fluorescence, and VBNC bacteria (third channel from the right), in which PI addition has no effect on mCherry red fluorescence (Fig. S1). The dynamics of intracellular pH for individual cells in each phenotype was measured by removing the background fluorescence, normalizing the pHluorin against the mCherry signal for each cell at each time point, and using a pH calibration standard (see Materials and Methods). Scale bar, 3 μm.
Decreasing bacterial acidity could help reduce antimicrobial resistance by eliminating bacteria that can survive being treated with antibiotics.
Scientists at the University of Exeter have developed a novel method that allows users to measure the pH of individual bacteria before, during and after treatment with antibiotics.
The research, published in the journal mBio, lays the foundation for understanding the special properties of bacteria that survive being treated with antibiotics, so that new ways of targeting them can be developed.
The Exeter University research team found that even before antibiotic treatment, common infection causing Escherichia coli cells that can survive treatment have a more acidic intracellular pH compared to clonal cells that are eliminated by the antibiotic treatment. These surviving cells are called persisters because they are responsible for persistent bacterial infections and contribute to antibiotic resistance.
Antibiotic resistance is one of the most pressing public health challenges and threatens the ability to effectively fight infectious diseases, with around 10 million people predicted to die annually of infections by 2050.
The University of Exeter research team has discovered the mechanisms that permit persisters to have an acidic pH. By measuring the genetic properties of these cells, they found that two cellular processes, namely tryptophan metabolism and carboxylic acid catabolism, are responsible for the low pH measured in persister bacteria.
Dr. Stefano Pagliara, a biophysicist in the Living Systems Institute, leading this research at the University of Exeter, said: "Our findings indicate that the manipulation of the intracellular pH represents a bacterial strategy for surviving antibiotic treatment. Our new data suggest a strategy for developing antibiotics that interfere with key cellular components of persisters and decrease their acidity."
The team is now working on expanding this research to find out whether cell acidity is key for antibiotic resistance in other critical bacterial pathogens such as Pseudomonas aeruginosa and Burkholderia pseudomallei and to identify drug molecules that can alter the pH of persister cells before antibiotic treatment.
"Persister Escherichia coli Cells Have a Lower Intracellular pH than Susceptible Cells but Maintain Their pH in Response to Antibiotic Treatment" is published on Tuesday, July 20th 2021.
More information: Olivia Goode et al, Persister Escherichia coli Cells Have a Lower Intracellular pH than Susceptible Cells but Maintain Their pH in Response to Antibiotic Treatment, mBio (2021). DOI: 10.1128/mBio.00909-21
Journal information: mBio
Provided by University of Exeter