New research suggests mineral nanoparticles as ubiquitous enzyme mimetics in Earth systems
Globally, the Earth system has thousands of terragrams (Tg) (1 Tg = 1012g) of mineral nanoparticles moving around the planet each year. These mineral nanoparticles are ubiquitously distributed throughout the atmosphere, oceans, waters, soils, in and/or on most living organisms, and even within proteins such as ferritin. In natural environments, mineral nanozymes can be produced by two pathways: 'top down' and 'bottom up' processes. Specifically, the weathering or human-promoted breakdown of bulk materials can result in nanomaterials directly (a top-down process), or nanomaterials can grow from precursors through crystallization, reaction, or biological roles (a bottom-up process).
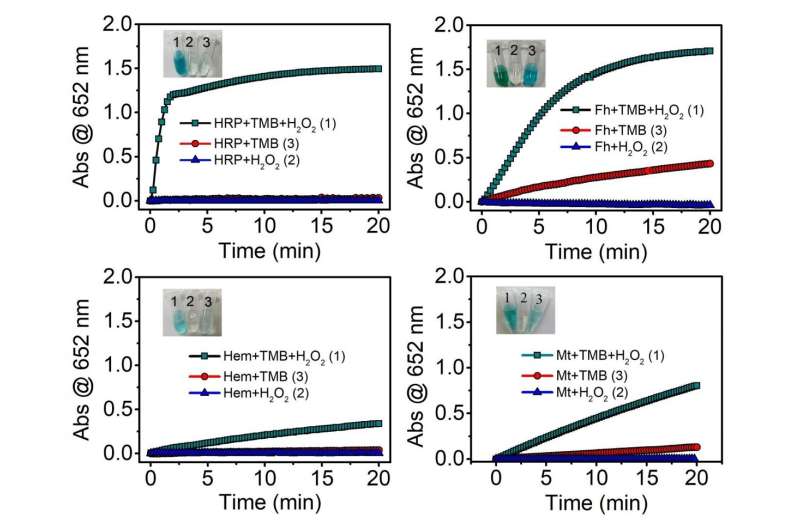
These mineral nanoparticles can possess multiple enzyme-like properties, e.g., oxidase, peroxidase, catalase, and superoxide dismutase, depending on the local environment. Iron-containing minerals, e.g., ferrihydrite, hematite, and magnetite, are ubiquitous in Earth systems and possess peroxidase-like activity. Among these iron (oxyhydr)oxides, ferrihydrite exhibited the highest peroxidase-like activity, owing to its smallest particle size and largest specific surface area. Because of the presence of ferrous iron, magnetite has considerably high peroxidase-like activity.
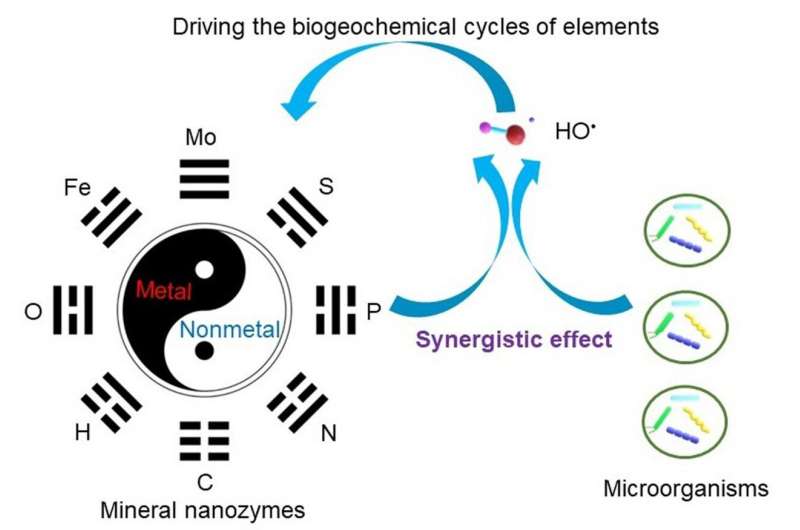
Compared with natural enzymes, mineral nanozymes show several advantages, such as low cost, increased stability, sustainable catalytic activity, and robustness to harsh environments. Because of their larger specific surface area, high ratios of surface atoms, wide band gap, and strong catalytic activities, mineral nanozymes play essential roles in biogeochemical cycles of elements in ecosystems.
Fungi and bacteria contribute approximately 70 Gt carbon (C) (1 Gt = 10 9t) and 120 Gt C to global biomass, respectively. Given that fungal hyphae can cumulatively extend hundreds of kilometers in soils kg-1 in environments such as the rhizosphere (i.e., 200-800 km kg-1) and that more than 94% of land plants and fungi form a symbiotic relationship, mineral nanozymes may have important implications in microbial-mineral coevolution, nutrient cycling in the surface Earth system, mineral carbon sequestration, and alleviation of global climate changes.
In Earth systems, taxonomically and functionally diverse microorganisms are a vast source of superoxide (O2-) or hydrogen peroxides (H2O2). These mineral nanozymes can regulate the levels of reactive oxygen species (ROS), including H2O2, O2- and hydroxyl radicals (HO+ ). By producing a strong oxidative HO+ , the interaction between mineral nanozymes and microorganisms may play an important role in driving the biogeochemical cycle of elements (Figure 2).
"All of the investigations on mineral nanozymes are still in the laboratory stage and are not field studies," said Guang-Hui Yu, a scientist at the School of Earth System Science, Tianjin University, in the Chinese city of Tianjin.
"The catalytic activity of mineral nanozymes is mainly determined by the oxygen vacancies (OVs) on the mineral surface", the researchers wrote in an article titled "Fungal Nanophase Particles Catalyze Iron Transformation for Oxidative Stress Removal and Iron Acquisition."
"These oxygen vacancies are often occupied by hydroxyl groups on the mineral surface," they explained.
Since mineral nanozymes can catalyze H2O2 to produce highly oxidizing HO+ , they have been extensively used in the field of environmental remediation. Compared with natural enzymes, mineral nanozymes can degrade organic pollutants in a wider pH range. For example, by degrading H2O2, Fe3O4 nanoparticles could effectively remove rhodamine B (RhB) in the pH range from 3.0 to 9.0.
"The effects of mineral nanozymes on microbial communities in the environment remain unclear," wrote the two researchers, "the findings of mineral nanozymes may have revealed a previously unknown feedback route of microbe-mineral coevolution that could shed light on a number of long-standing questions, such as the origin and evolution of life by modulating ROS levels."
These two scholars likewise revealed in the study, which was published in the Science China Earth Sciences, that the discovery of nanomaterials as new enzyme mimetics has changed the traditional idea that nanomaterials are chemically inert in Earth systems. Given the terragram (Tg)-level abundance of mineral nanoparticles in Earth systems, it is statistically highly probable for some of them, particularly those of biotic origin, to behave as mineral nanozymes to catalyze superoxide and H2O2 and promote the biogeochemical cycles of oxygen and other elements.
More information: Zhi-Lai Chi et al, Nanozyme-mediated elemental biogeochemical cycling and environmental effects, Science China Earth Sciences (2021). DOI: 10.1007/s11430-020-9756-5
Journal information: Science China Earth Sciences
Provided by Science China Press