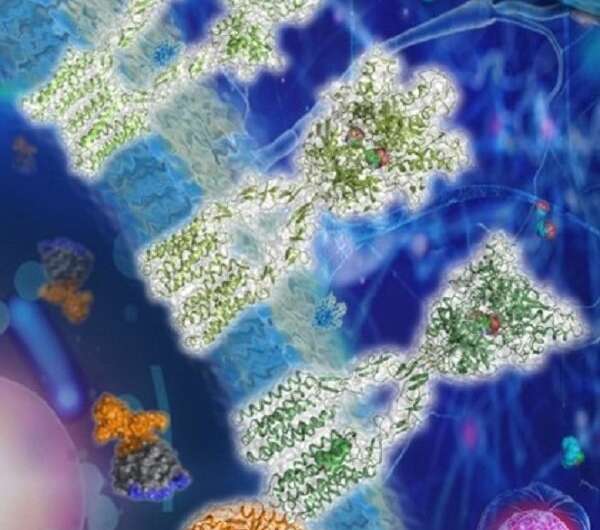
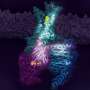
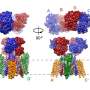
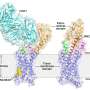
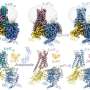
Structures of mGlu2 in different conformational states. Credit: Dr. WU Beili’s laboratory
Metabotropic glutamate receptors (mGlus), which belong to class C G-protein-coupled receptor (GPCR) family, play key roles in modulating neuronal excitability and synaptic transmission in the central nervous system. The mGlus (mGlu1-8) identified in humans serve as therapeutic targets for a variety of neurological and psychiatric disorders such as Parkinson's disease, Alzheimer's disease and schizophrenia, but drugs targeting the mGlus are not currently available.
In two back-to-back studies published in Nature, a team of researchers led by Wu Beili, Zhao Qiang, Wang Mingwei and Liu Hong at the Shanghai Institute of Materia Medica of the Chinese Academy of Sciences (CAS), collaborating with research groups led by Sun Fei at Institute of Biophysics of CAS and by Liu Jianfeng from Huazhong University of Science and Technology, respectively, determined six cryo-electron microscopy (cryo-EM) structures of several mGlus in distinct functional and dimeric states, which offers opportunities to develop new treatment for neurological and psychiatric diseases.
The researchers solved the structures of mGlu2 and mGlu7 homodimers in the inactive state; the structures revealed distinct dimerization modes. Using mutagenesis and cell signaling assays, the researchers found that the transmembrane domain (TMD) dimer interface in the inactive mGlu2 homodimer is subtype-specific and has a critical role in stabilizing the inactive conformation of the receptor. They determined the structures of mGlu2 homodimer in the inactive, intermediate activation (Gi protein-free) and fully activate (Gi protein-bound) states.
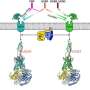
The structure of mGlu4 in complex with the Gi protein reveals a similar dimeric association mode, suggesting that different mGlus may modulate receptor activation in a similar manner.
Additionally, the researchers found that extracellular domains (ECDs) of both subunits are occupied by the agonist, but only one TMD is capable of coupling to G protein. The asymmetric dimer association provides different interaction environments for the two subunits and may cause distinct conformational changes within the two TMDs, which only allow one subunit to recognize the G protein. The G protein then forms a spatial hindrance to block the G protein coupling by the other subunit.
The studies offer the first thorough view of receptor conformational changes during the process of mGlu activation and give valuable insights into asymmetric activation of mGlus. They highlight the importance of cooperativity between different subunits as well as between different receptor domains in controlling receptor function, thereby expanding the knowledge about signal transduction of class C GPCRs.
More information: Shuling Lin et al, Structures of Gi-bound metabotropic glutamate receptors mGlu2 and mGlu4, Nature (2021). DOI: 10.1038/s41586-021-03495-2
Juan Du et al, Structures of human mGlu2 and mGlu7 homo- and heterodimers, Nature (2021). DOI: 10.1038/s41586-021-03641-w
Journal information: Nature
Provided by Chinese Academy of Sciences