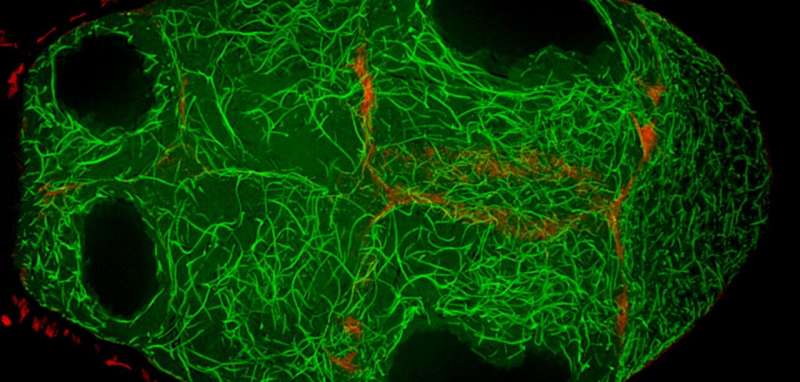
Microtubules (green) are bound with actin (red) through the shortstop protein, creating bridges on which motor proteins transport materials to the oocyte. Credit: Northwestern University
A protein helps direct the flow of materials in the Drosophila ovary during the development of egg cells, according to a Northwestern Medicine study published in Current Biology.
The protein, called Short Stop, helps organize microtubules that carry proteins, RNA and even whole organelles from nurse cells to the oocyte, which cannot make its own.
"This is part of the big question: What is the mechanism that moves things from the nurse cells to the oocyte?" said Vladimir Gelfand, Ph.D., the Leslie B. Arey Professor of Cell, Molecular, and Anatomical Sciences and senior author of the study.
Egg cells, also known as oocytes, arise from stem cells very early in an organism's life. During the oocyte's development, specialized "nurse cells" pump proteins, messenger RNA (mRNA) and more into the oocyte, giving the oocyte materials to build structures necessary for organism development.
"They share organelles, they share everything," said Gelfand, who is also a professor of Cell and Developmental Biology and a member of the Robert H. Lurie Comprehensive Cancer Center.
Transport from the nurse cells to the oocyte occurs through specialized ring canals, but only in one direction. The mechanisms that underlie this directionality have previously been unknown, according to the authors.
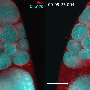
Oocyte complex, mitochondria (red) moving from nurse cells on the left to the oocyte on the far right. Credit: Northwestern University
In the study, Wen Lu, Ph.D., research associate in the Gelfand laboratory and lead author of the study, used RNA interference (RNAi) to selectively knock down specific genes involved in transport in Drosophila fruit flies, searching for one that—when deleted—would affect the size of the oocyte.
Lu found that the protein Short Stop was crucial for unidirectional transport. The protein links actin filaments on the nurse cell side with microtubules growing from the oocyte side, helping to organize microtubules that serve as tracks allowing motor proteins to carry proteins and organelles across.
Importantly, those microtubules are polarized, arranged with the plus ends towards the nurse cells and the minus ends towards the oocyte. The specific motor protein, cytoplasmic dynein, that transports materials to the oocyte is coded to run towards the minus ends, ensuring transport in the correct direction.
However, when Short Stop was deleted, microtubule polarity is affected, causing bi-directional transport and limiting growth of the oocyte.
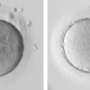
Close-up of mitochondria moving through ring canals. Credit: Northwestern University
"It's like building a highway that only goes one way, but if we delete Short Stop it becomes a two-way street," Lu said. "The oocyte is receiving some materials, but it's also giving some up—so its net growth is zero."
This mechanism is also present in mammals, according to Gelfand, so studying these mechanisms in flies will help the scientific community better understand the complex mechanisms that govern oocyte development in humans.
Next, Gelfand said the laboratory plans to examine the motor proteins that transport materials from the nurse cells to the oocyte.
More information: Wen Lu et al, Gatekeeper function for Short stop at the ring canals of the Drosophila ovary, Current Biology (2021). DOI: 10.1016/j.cub.2021.05.010
Journal information: Current Biology
Provided by Northwestern University