Cellular mechanisms of early mammary gland development unraveled
Despite long-standing interest, the cellular mechanisms driving the initiation of mammary gland development have remained elusive for decades, mostly due to technical limitations in studying dynamic cell behaviors in live tissues. Recent advances in microscopic methods and availability of various mouse models allowed the research group of Marja Mikkola from HiLIFE Institute of Biotechnology, University of Helsinki to address this question. This is the first time live tissue imaging has been used to visualize the emergence of the mammary gland.
The mammary gland is the class-defining organ of mammals, yet we know surprisingly little about how its development commences. In their recent study published in the Journal of Cell Biology, the research group used time-lapse imaging to show that the growth of the mammary bud is primarily fueled by migration of cells to the bud. In contrast, although increase in cellular size and cell proliferation contribute to this process, the role of these mechanisms remains minor.
"Interestingly, mammary bud cells, unlike most of other skin derivatives such as hair follicle and tooth bud, do not divide for several days, indicating that this might be a unique feature of early mammary gland development" says graduate student Ewelina Trela, the lead author of the study. "However, we do not yet know why this happens," she continues.
Mammary buds use a previously undescribed mechanism for invagination
Tissue invagination, or tissue folding inwards into the underlying stroma, is a fundamental mechanism that occurs to generate the architecture of many organs. In the same piece of work, the authors describe a novel mechanism for tissue invagination.
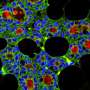
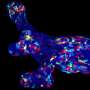
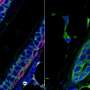
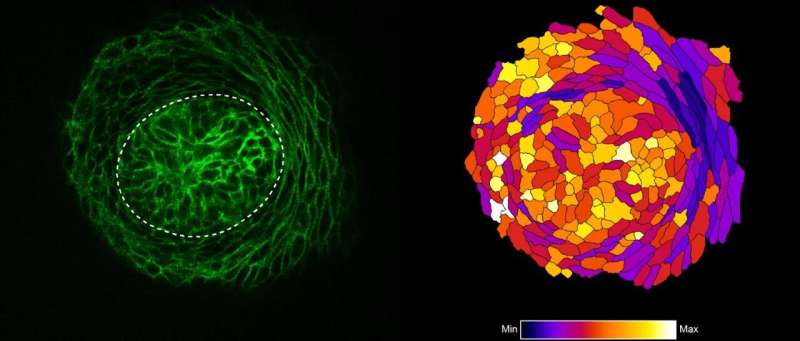
"Using confocal fluorescence microscopy, we found thin and elongated epidermal keratinocytes surrounding mammary bud in a rim like fashion: their appearance and disappearance coincided with the invagination process suggesting that these cells, named ring cells, could be functionally important" details principal investigator Mikkola.
Next, the Mikkola group teamed up with the group of Sara Wickström at HiLIFE and Faculty of Medicine, University of Helsinki to establish live imaging of the forming mammary bud, which confirmed that ring cells move circumferentially around the mammary bud.
The study also revealed that the ring cells exert contractile force through the actomyosin network, via non-muscle myosin IIA (NMIIA). The functionality of ring cells was impaired in NMIIA deficient mice leading to compromised mammary bud shape. Whether other developing organs utilize a similar cellular mechanism for invagination remains an open question.
More information: Ewelina Trela et al, Cell influx and contractile actomyosin force drive mammary bud growth and invagination, Journal of Cell Biology (2021). DOI: 10.1083/jcb.202008062
Journal information: Journal of Cell Biology
Provided by University of Helsinki