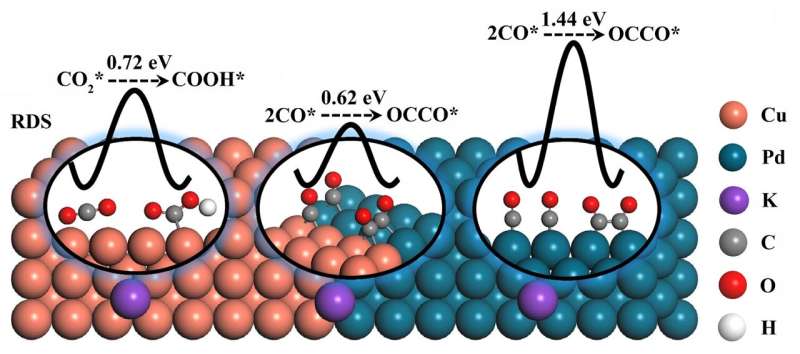
An intimate CuPd(100) interface was designed to lower the energy barriers of intermediate reaction (CO2* hydrogenation and C-C coupling) and improve the efficiency of C2 products. The optimal CuPd(100) interface catalyst exhibited a C2 Faradaic efficiency of 50.3%, which was 2.1 times higher than that of Cu catalyst (23.6%) at ?1.4 VRHE in 0.1 M KHCO3. Credit: Chinese Journal of Catalysis
Using intermittent electric energy to convert excessive CO2 into C2 products, such as ethylene and ethanol, is an effective strategy to mitigate the greenhouse effect. Copper (Cu) is the only single metal catalyst which can electrochemically convert CO2 into C2 products, albeit with undesirable selectivity of the C2 product. Therefore, improving the conversion efficiency of Cu-based catalysts for reducing CO2 to C2 products has attracted great attention.
Recently, a research team led by Prof. Min Liu from Central South University, China, designed a Cu-Pd bimetallic electrocatalyst with a CuPd(100) interface that can lower the energy barrier of C2 product generation. The electrocatalyst was obtained via an in-situ growth method based on thermal reduction to afford Pd nanoparticles as nucleated seeds. The results were published in Chinese Journal of Catalysis.
Generally, there are two limiting factors for achieving the electroreduction of CO2 to C2 products, namely the amount of CO* intermediate (* indicates the intermediate is adsorbed on the surface of the catalyst) and the C-C coupling step (generally two CO* coupling). For Cu catalysts, the energy barrier of the C-C coupling step is relatively low. However, the CO2 adsorption and CO2* hydrogenation ability of Cu are unfavorable, resulting in an insufficient amount of CO* involved in the subsequent C-C coupling step. Palladium (Pd) is an efficient catalyst that exhibited strong CO2 adsorption and ultrafast reaction kinetics for CO* formation. However, CO* poisoning on the Pd surface makes it unsuitable for generating C2 products. To take full advantage of both Cu (low energy barrier of C-C coupling) and Pd (ultrafast kinetics for CO* formation), the assembly of a CuPd bimetallic catalyst was envisaged as a potential method for optimizing the efficiency of C2 product formation.
The density functional theory (DFT) calculation shows that the CuPd (100) interface enhanced the adsorption of CO2 and reduced the energy barrier of CO2* hydrogenation step; thus, sufficient CO* participated in the C-C coupling reaction. In addition, the energy barrier of rate-determining step for C2 product generation on CuPd (100) interface is 0.61 eV, which is lower than that on Cu(100) surface (0.72 eV).
Then the target CuPd (100) interface catalyst was prepared by a simple wet chemical method and proved by different characterization methods. The temperature programmed desorption and gas sensor experiment results proved the enhanced CO2 adsorption and CO2* hydrogenation ability on CuPd(100) interface, respectively. As a result, the CuPd(100) interface catalyst exhibited a C2 Faradaic efficiency of 50.3%, which was 2.1 times higher than that of Cu catalyst (23.6%) at -1.4 VRHE in 0.1 M KHCO3. This work provides a reference for the rational design of Cu-based electrocatalyst for CO2 electroreduction by adjusting the intermediate reaction energy barrier.
More information: Li Zhu et al, Tuning the intermediate reaction barriers by a CuPd catalyst to improve the selectivity of CO2 electroreduction to C2 products, Chinese Journal of Catalysis (2021). DOI: 10.1016/S1872-2067(20)63754-8
Provided by Chinese Academy Sciences