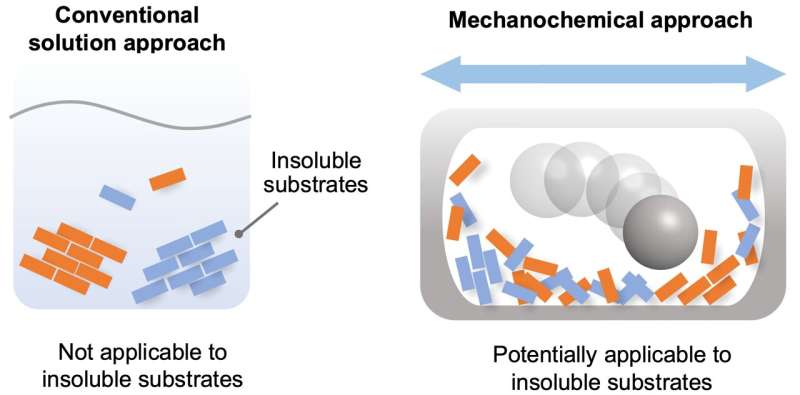
Insoluble reactants are hardly reactive in solution, but may react in solvent-free systems using ball milling to drive chemical reactions in the solid state. Credit: Tamae Seo, et al. Journal of the American Chemical Society. March 30, 2021
Scientists from Hokkaido University have developed a rapid, efficient protocol for cross-coupling reactions, vastly expanding the pool of chemicals that can be used for the synthesis of useful organic compounds.
Chemical reactions are a vital process in the synthesis of products for a diversity of purposes. For the most part, these reactions are carried out in the liquid phase, by dissolving the reactants in a solvent. However, there are a significant number of chemicals that are partially or completely insoluble, and thus have not been used for synthesis. The starting materials required for the synthesis of many cutting-edge organic materials—such as organic semiconductors and luminescent materials—are often poorly soluble, leading to problems in solution-based synthesis. Therefore, the development of a solvent-independent synthetic approach to overcome these long-standing solubility issues in organic synthesis is highly desired to synthesize new valuable organic molecules.
In recent years, synthetic techniques using ball milling have been used to carry out solvent-free reactions in the solid phase. It has been proposed that the use of ball milling would potentially overcome the aforementioned solubility issues in synthetic chemistry, but a systematic study for such purpose has never been carried out.
A team of four scientists from Hokkaido University's Institute for Chemical Reaction Design and Discovery (WPI-ICReDD), led by Associate Professor Koji Kubota and Professor Hajime Ito, have developed a rapid, efficient, solvent-free protocol for Suzuki−Miyaura cross-coupling reaction of insoluble aryl halides. The protocol was published in the Journal of the American Chemical Society.
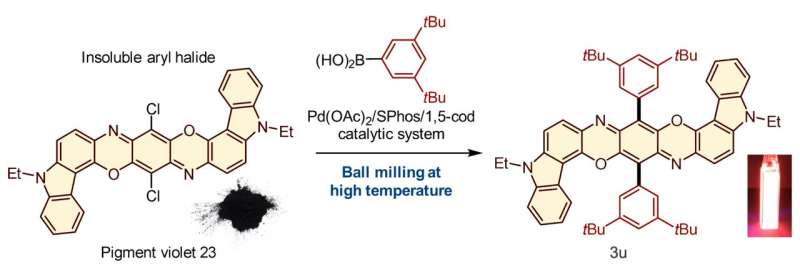
When pigment violet 23, one of the mostly-insoluble aryl halides, was subjected to the reaction with an aryl boron nucleophile at 120 °C in a ball mill in the presence of the palladium-based catalytic system, it was converted to a product 3u that was strongly photoluminescent when dissolved in dichloromethane. Credit: Tamae Seo, et al. Journal of the American Chemical Society. March 30, 2021
Aryl halides are popular starting materials for the synthesis of organic functional molecules, primarily by the palladium-catalyzed Suzuki-Miyaura cross-coupling reaction—for which Hokkaido University's Professor Emeritus Akira Suzuki shared the 2010 Nobel Prize in Chemistry. Although the cross-coupling reactions have been employed for the synthesis of a wide range of valuable molecules, insoluble aryl halides are not suitable substrates because Suzuki-Miyaura cross-coupling reactions have primarily been carried out in solution.
Given this limitation, the scientists focused on the development of an efficient solid-state Suzuki-Miyaura cross-coupling of a number of extremely unreactive insoluble aryl halides. The key equipment consisted of a ball mill, for mixing the reactants; a heat gun, to increase the temperature at which the reactions took place; and the use of a catalytic system composed of palladium acetate (the catalyst), SPhos (a high-performance ligand for Suzuki−Miyaura cross-coupling reactions) and 1,5-cyclooctadiene (dispersant and stabilizer).
The capstone of this study was the application of the solvent-free solid-state reaction to mostly-insoluble aryl halides. These reactants did not yield any products in conventional solution-based reactions. The solid-state reactions using the high-temperature ball milling, however, gave the desired products. Importantly, the team discovered a new strong photoluminescence material prepared from insoluble pigment violet 23.
"The high-temperature ball-milling technique and our catalytic system are essential for these cross-coupling reactions of insoluble aryl halides, and the protocol we have developed expands the diversity of organic molecules derived from insoluble starting materials," says Koji Kubota.
More information: Tamae Seo et al. Tackling Solubility Issues in Organic Synthesis: Solid-State Cross-Coupling of Insoluble Aryl Halides, Journal of the American Chemical Society (2021). DOI: 10.1021/jacs.1c00906
Journal information: Journal of the American Chemical Society
Provided by Hokkaido University