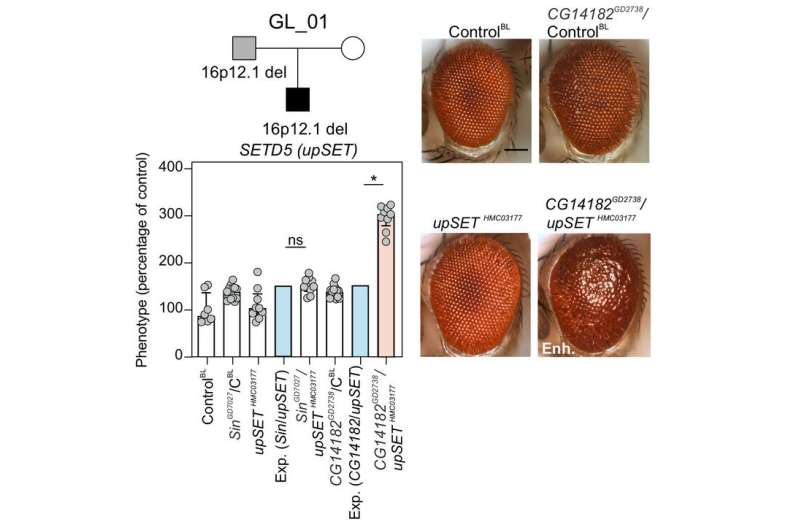
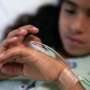
“Second-hits” may modulate impact of a large deletion on human chromosome 16 associated with neurodevelopmental disorders through genetic interactions. (top left) A pedigree of an individual carrying the developmental-delay-associated 16p12.1 deletion (inherited from the father) and a de novo mutation in the intellectual disability-associated SETD5 gene. (bottom left, right) upSET, the fly version of the human SETD5 gene, synergistically interacts with CG14182 (fly version of the human gene MOSMO located within the chromosome 16 deletion) resulting in more severe neurodevelopmental defects in the fly. Credit: Lucilla Pizzo, Penn State
A mutation that deletes a large segment of human chromosome 16 and is associated with neurodevelopmental disorders doesn't work alone. A new functional assay demonstrates how the deletion could be sensitizing the genome such that "second hits"—mutations in other parts of the genome—may genetically interact with the deletion to exacerbate the symptoms. Different second hit mutations found in different individuals could also help explain the variability seen in symptoms associated with the deletion.
A paper describing the research, by a team led by Penn State scientists, appears online in the journal PLOS Genetics.
"Large genetic deletions like this one on chromosome 16 are associated with many neurodevelopmental disorders, including autism, schizophrenia and intellectual disability," said Santhosh Girirajan, associate professor of genomics at Penn State and the leader of the research team. "In most cases when we see a deletion like this in an individual with a neurodevelopmental disorder, it gets treated like a smoking gun. We assume it is the cause of the disorder and stop looking for any other potential contributing genetic factors, but this deletion is different."
The deletion on human chromosome 16, referred to as 16p12.1, spans more than half a million base pairs of DNA and contains seven genes. It has been associated with several neurodevelopmental disorders, including intellectual disability/developmental delay (ID/DD), schizophrenia and epilepsy. However, it is different than many other large deletions because, in most cases, individuals identified as having the deletion inherited it from a parent who was never diagnosed with a neurodevelopmental disorder.
"Because these types of mutations often cause severe symptoms, they are not usually passed from parent to child, but instead occur 'de novo'—as a new mutation—in the individual that is diagnosed," said Lucilla Pizzo, a graduate student at Penn State and first author of the paper. "We have evidence that parents who pass this deletion on to their children may be mildly affected, but mostly they fly under the radar medically speaking. This led us to think that there may be other new mutations second hits—occurring in the diagnosed individuals that interact with the deletion to contribute to the disorders."
The research team set out to test this "two-hit" model in the laboratory by simultaneously reducing the expression level of genes in the deletion with potential second hit genes in two different model systems—fruit fly (Drosophila melanogaster) and frog (Xenopus laevis). Of the seven genes in the 16p12.1 deletion, the team identified four that were shared in both the fly and the frog. The majority of the experiments were done in flies because it is easier, faster, and cheaper, and then validated using the frog.
"In flies, we can experimentally 'knock down'—or reduce the level of—genes found in the deletion one at a time," said Girirajan. "This mimics the reduction that occurs when an individual has the deletion on one of their two copies of chromosome 16 and allows us to see the individual impact of the genes on the fly. We can also do this for potential second hit genes. Finally, we can cross any two flies that have different genes knocked down to see if the two genes interact."
The team tested the interaction of genes found in the 16p12.1 with each other, with other genes known to be involved in neurodevelopment, and with potential second hit genes identified as rare mutations by sequencing the genomes of individuals diagnosed with neurodevelopmental disorders who have the chromosome 16 deletion.
"We are looking for situations where the effects of the two knockdowns don't just add up like one plus one equals two," said Pizzo. "Interactions between genes could strengthen the effects—one plus one equals five, for example—or even reduce it—one plus one equals zero."
The team found very few interactions among the four genes found in the deletion with each other—a very different result than they had previously found for another large deletion on human chromosome 16 where interactions were stronger between genes within the deletion than with genes outside of the deletion. Instead, they found that genes in the 16p12.1 deletion interacted more strongly with genes known to be involved in neurodevelopment and that second-hit genes modulated the impact of a 16p12.1 gene by genetic interactions in half of the combinations tested.
"We were surprised that the genes in the 16p12.1 deletion seemed to behave so differently than genes in the other deletions that we have studied, but as with most things in genetics, it's never as simple as we hope it to be," said Girirajan. "In a clinical setting, when we see a deletion like this in an individual with a neurological disorder, we want to be able to say that the deletion is the cause. For example, it makes thinking about potential treatments simpler. But, our result points to the importance of looking beyond the easy answer. Also, because different individuals carry different second hit genes, it could help us to understand the variability of symptoms in individuals with the deletion."
More information: Lucilla Pizzo et al. Functional assessment of the "two-hit" model for neurodevelopmental defects in Drosophila and X. laevis, PLOS Genetics (2021). DOI: 10.1371/journal.pgen.1009112
Journal information: PLoS Genetics
Provided by Pennsylvania State University