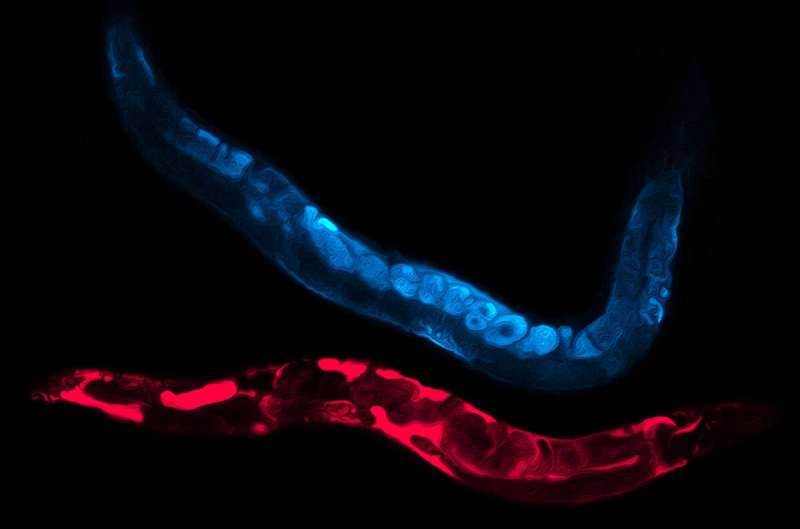
This color-manipulated image features a blue “control” worm and a magenta worm that has been exposed to chronic heat stress. The chronic heat stress caused the yolk protein to accumulate in the magenta worm's body cavity as indicated by the dark pools. In the blue-colored control worm, the yolk protein has been properly trafficked into the eggs, as shown in the round shapes. Credit: Rosemary Plagens.
Chronic stress caused by changing temperatures, Alzheimer's disease or other diseases and forces is an unavoidable part of life. At the cellular level, the "first responders" to stress are known as molecular chaperones. Yet just as a major health crisis may be more than a team of paramedics can handle, a new Florida Tech study examining chronic stress in the Caenorhabditis elegans roundworm shows that major stress events overwhelm molecular chaperones, leading to a variety of problems, including a defect in how proteins are transported between cells.
This cellular trafficking defect has been seen in cell culture models for neurodegenerative disease, but this is the first time this defect has been demonstrated with stress and in an intact organism.
The research, "Chronic temperature stress inhibits reproduction and disrupts endocytosis via chaperone titration in Caenorhabditis elegans," was published in the journal BMC Biology on April 15 by Florida Tech biologists Rosemary Plagens, a Ph.D. student, Isiah Mossiah, a master's student, and faculty members Karen Kim Guisbert and Eric Guisbert.
Worms exposed to chronic temperature stress—in this case, heat—can move and eat but can no longer lay eggs. Using a combination of genomic, cellular and molecular tools, the Florida Tech team found that in cases of chronic stress, molecular chaperones become overwhelmed, leading to problems with protein trafficking between cells in different tissues and contributing to the decline in egg laying during the chronic stress. This is the first time the trafficking defect has been found in cells faced with heat stress, as opposed to the stress caused by genetic mutations associated with neurodegenerative diseases.
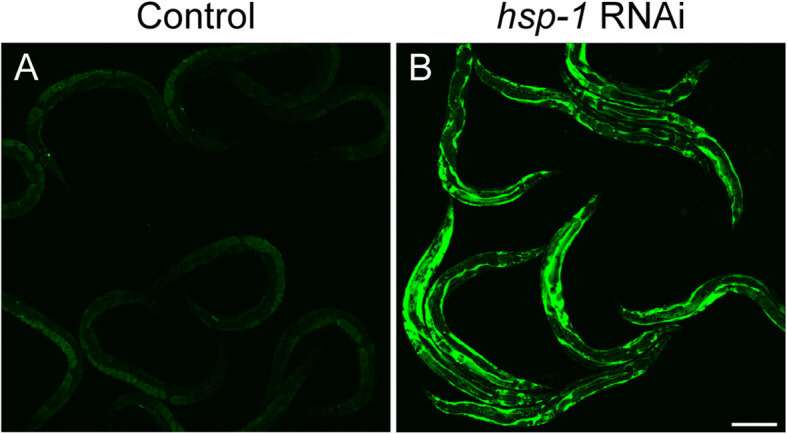
hsp-1 knockdown inhibits yolk endocytosis. hsp-1 knockdown causes yolk to accumulate in the pseudocoelomic space, as shown in YP170::GFP (RT130) worms that were raised to the L4 stage on OP50 and then exposed to either a control (L4440, empty vector) or b hsp-1 RNAi for 48 h at 20 °C. Shown are representative images from three independent trials with n ≥ 10 worms. Scale bar: 150 μm. Credit: BMC Biology (2021). DOI: 10.1186/s12915-021-01008-1
"The problems in protein trafficking arise because chaperones have two primary jobs: addressing damaged proteins and assisting in protein trafficking," said Plagens, first author on the study. "We believe that chronic heat stress creates so much protein damage that the chaperones are too busy with repairs to also help with protein trafficking."
The researchers also found that increasing the number of chaperones in the organism can reverse some of the effects of chronic heat stress, suggesting that this is a promising area for further research into the relationship between genetics and the environment to combat human diseases.
"Understanding how chronic stress affects an animal from its behavior all the way down to the molecules in its cells is not only fascinating but also very important in the context of studying chronic diseases," Plagens said.
More information: Rosemary N. Plagens et al. Chronic temperature stress inhibits reproduction and disrupts endocytosis via chaperone titration in Caenorhabditis elegans, BMC Biology (2021). DOI: 10.1186/s12915-021-01008-1
Journal information: BMC Biology
Provided by Florida Institute of Technology