Cancer drugs could be delivered in molecular cages unlocked by light
Molecular cages created by Imperial researchers could lead to more targeted cancer drug delivery, causing greater efficiency and fewer side effects.
Many drugs, including cancer therapies, can break down in the body, which reduces their efficacy and can mean more doses are needed. They can also cause side effects when they damage healthy tissues.
Researchers are therefore searching for ways to make drugs more targeted, so they only start acting when they reach the right part of the body, such as the site of a cancer tumor.
Now, researchers from the Department of Chemistry at Imperial College London have created a novel type of 'cage' for a molecule with anticancer properties. The release of the molecule from the cage can be then controlled by external stimuli, such as light. The study is published today in Angewandte Chemie.
Ph.D. student Timothy Kench said: "We're really excited by the approach. By regulating the biological activity of small molecules, we can design improved therapies or study specific cellular processes."
Trapping drug molecules
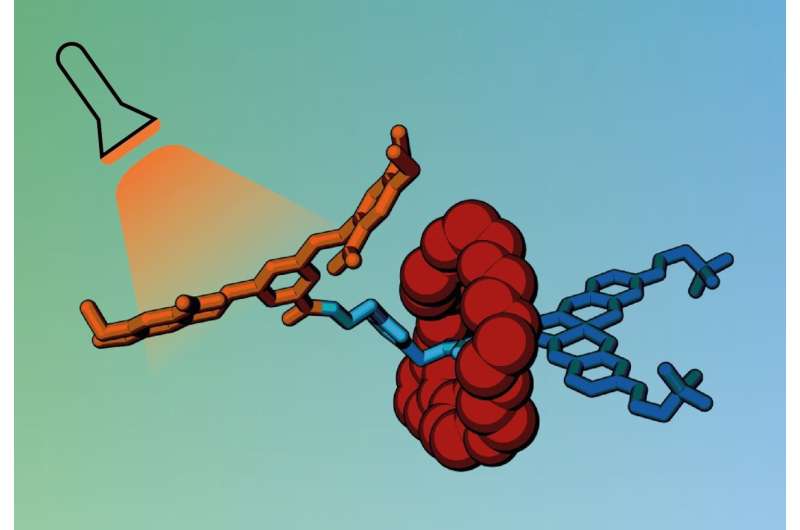
The new cage works by 'trapping' drug molecules within a non-toxic carrier that can transport the drug to the required site before it is released. The cage consists of bulky molecular groups that wrap around the drug, blocking its biological activity until they are detached through application of a trigger.
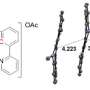
To make the cage, the team used a particular type of molecule called a rotaxane. Rotaxanes have a molecular ring trapped on a dumbbell-shaped component, called an axle, which has stopper groups at either end to prevent the ring from slipping off. The ring acts like a molecular shield, blocking access to the axle and preventing it from interacting with other molecules.
The researchers designed a rotaxane with an axle including a biologically active molecule that normally kills cancer cells by interacting with their DNA. While the ring is present, the active molecule cannot bind to DNA, shutting off its toxicity.
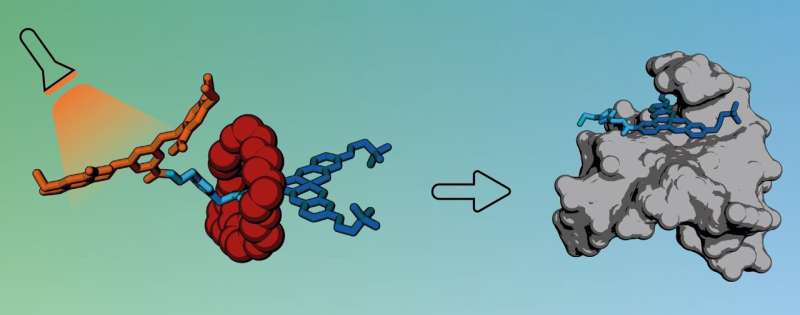
However, when exposed to light or a specific enzyme, one end of the axle breaks off, releasing the ring and allowing the active molecule to bind to DNA in cancer cells.
Targeting cancer
The active molecule incorporated into the rotaxane is particularly good at interacting with a special type of DNA structure called a G-quadruplex (G4). Due to the biological roles these DNA structures play in cells, they have been proposed as potential drug targets for cancer, giving scientists hope that compounds which can interact with G4s could be used in the future as new anticancer medicines.
The researchers first tested their new rotaxane drug carrier using strands of DNA extracted from cells and found no interaction at all, confirming that the rotaxane's ring was blocking access to the active compound.
Next, they tested their rotaxane in live cancer cells, first showing that the rotaxane loaded with the active compound was not toxic to these cells under normal conditions. When exposed to light, however, nearly all the cancer cells were dead within a few hours, demonstrating that the active compound could be released within the targeted cancer cells in a highly controlled manner.
Tracking the rotaxane in the cancer cells using confocal microscopy showed that before shining light it remained in the outer parts of the cell, which contain no DNA. After light was shone on the cells, however, the released active molecule moved to the nucleus, where the majority of the DNA in cells is stored. These experiments suggested that it is the triggered binding to DNA that caused the cancer cells to die.
Professor Ramon Vilar said: "Being able to deliver drugs at the right place and at the right time is an important challenge in medicinal chemistry. Our research shows that it is possible to achieve this by caging active molecules in rotaxanes."
Click chemistry
While light is a good trigger in terms of how well its location and intensity can be controlled, in practical use it would be limited to skin cancers or potentially those that can be reached inside the body with an endoscope. The researchers are therefore also testing the possibility of releasing the rotaxane ring with specific enzymes, such as those found in abundance only in cancer cells. Dr. Jamie Lewis said:
"'Click reactions," which were used to prepare these rotaxanes, are easy and modular reactions that join up building blocks, like a molecular Lego kit. This is great because you can 'click' all sorts of different molecules together, making our approach very general and adaptable."
The modularity of their approach would allow researchers to use a different anti-cancer molecule or introduce an alternative mechanism for activation. Effectively, researchers could just choose the components they want and click them together using the same process.
More information: Timothy Kench et al. Rotaxanes as Cages to Control DNA Binding, Cytotoxicity, and Cellular Uptake of a Small Molecule, Angewandte Chemie International Edition (2021). DOI: 10.1002/anie.202100151
Provided by Imperial College London