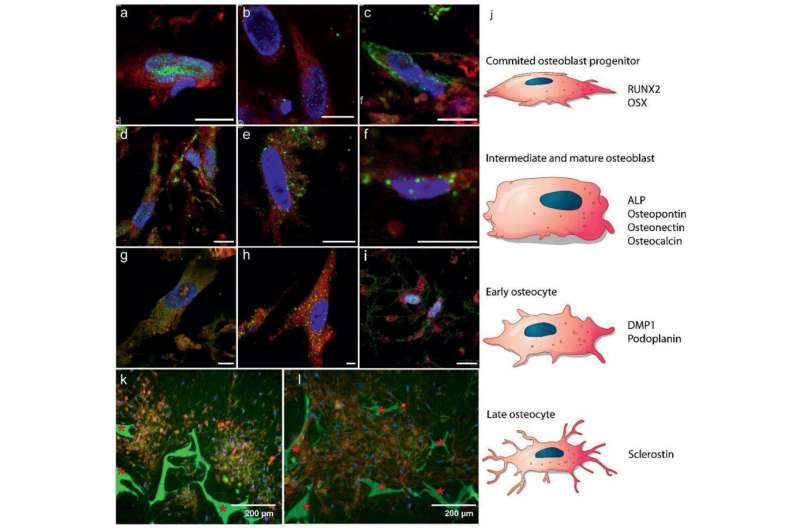
Differentiation of hBMSCs into osteoblasts and osteocytes: a–i) Fluorescence immunohistochemistry imaging showing markers for a–c) early stages of osteoblast formation, d–f) mature osteoblasts, and g–i) osteocyte development (5.55 mm glucose). Color code: red – cell cytoplasm, blue – cell nuclei, green: a) RUNX2 (day 7), b) OSX (day 7), c) ALP (day 26), d) osteocalcin (day 26), e) osteopontin (day 26), f) osteonectin (day 21), g) DMP1 (day 28), h) podoplanin (day 28), and i) sclerostin (day 28). Scale bars: 10 µm. See Figure S4, Supporting Information, for separate channels. j) Schematic illustration of MSCs differentiation into osteoblasts and osteocytes, indicating at which state which protein expression is expected in a–i. k,l) Fluorescent images indicating self‐organized domains of osteocytes embedded in a mineralized matrix after 8 weeks (25 mm glucose), k) co‐localization of osteocytes (sclerostin, red) and mineral (calcein, green), and l) collagen (CNA35, red) and mineral (calcein, green) * Indicates the silk fibroin scaffold.
Imagine using stem cells from your bone marrow to grow a piece of bone tissue in the lab, after which medical doctors explore which drugs have the desired effect on your bones. In this way, a tailor-made treatment plan would be made for everyone, with the best approach being clear in advance: personalized medicine at its best.
That vision of the future is no longer science fiction now that researchers from Eindhoven University of Technology and Radboud university medical center have actually realized the first part: growing a lifelike piece of bone tissue from human stem cells. It is the first organoid of bone, a simplified version of the original, the researchers report today in the journal Advanced Functional Materials.
Coherent picture
"With this, we present, for the first time, the full picture of early-stage bone formation," says Sandra Hofmann, associate professor in Bioengineering Bone from TU/e. And that is of great importance: how our bones are formed is still largely a mystery. Bone is a very complex material in which countless cells and processes interact, and comprises an ingenious matrix of collagen and mineral to provide strength. Much is known about the individual components, but a coherent picture has been lacking until now.
Three types of cells play the main role in bone formation: osteoblasts (which build bone tissue), osteoclasts (which take bone away) and osteocytes (which regulate the building and breakdown of bone). "Most studies so far have focused on one of these types of cells, but that is not a good representation of the real tissue," says Hofmann. "We present here a piece of woven bone (early-stage bone) that developed from stem cells and contains two types of these cells: osteoblasts and osteocytes. We now see that we can make lifelike bone exclusively with these two cell types."
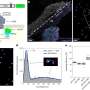
ECM development: a) 3D FIB/SEM reconstruction shows the embedding of the cells in the collagen matrix (cyan). Discrete cells are represented with different colors. b) TEM image of a 70 nm section showing the random distribution of collagen fibrils. Collagen type I was identified by immunolabelling. c–e) Fluorescent immunohistochemistry identifying key non‐collagenous proteins in the collagenous matrix: c) Co‐localization of osteocalcin (green) and collagen (red). d) Osteopontin (green) distribution in the collagen matrix (red). * Indicates the silk fibroin scaffold. e) Co‐localization of DMP1 (green) with the collagen structure (see Figure S5, Supporting Information, for collagen image). f–g) Raman microspectrometry of mineralized matrices. f) Localized Raman spectra of mineralized collagen of developing zebrafish bone (red), the 3D osteogenic co‐culture (blue), and human bone of a 10 year old female (grey g) Raman derived mineral/matrix ratios of 4 mineralized tissues of zebrafish (N = 6, red), Osteogenic 3D culture (N = 7, green), 10 year old human female (N = 1, grey), and 48+ years old human male (N = 7, black, taken from ref. [36]). Bars indicate sample standard deviations. h) Heat map presentation of a 3D FIB/SEM cross section showing disorganized collagen fibrils with different degrees of mineralization (Figure S10, Supporting Information). Arrowheads indicate non‐mineralized collagen fibrils (light blue), arrow indicates mineralized collagen fibril (orange). i) TEM image showing individual mineralized collagen fibrils.
Getting wiser from molecular poking
"And perhaps more importantly, our system behaves just like early-stage bone," says Anat Akiva, assistant professor Cell Biology at Radboudumc. "We show that both types of cells produce the proteins that they need for their functionality, and we show with the greatest detail that the matrix actually is the bone matrix we see in real tissue."
The fact that a simplified representation of the formation of bone at the molecular level is now possible offers unprecedented possibilities, according to the researchers. "A bone consists of 99% collagen and minerals, but there is also another 1% of proteins that are essential for successful bone formation," explains professor Nico Sommerdijk from Radboudumc. "So what's the role of these proteins? How do they support bone formation? Never before have we been able to look at the milestones of this process at a molecular level."
And with that, they immediately have a good entrance to investigate the cause of genetic bone diseases such as "brittle bone disease" and their possible treatments. "Remember that the origin of many diseases is at the molecular level—and so is the treatment," says Akiva. "In fact, we now have a simple system in a reliable environment in which we can poke around and see how bone cells react to the stimuli we provide."
More information: Anat Akiva et al, An Organoid for Woven Bone, Advanced Functional Materials (2021). DOI: 10.1002/adfm.202010524
Journal information: Advanced Functional Materials
Provided by Eindhoven University of Technology
![ECM development: a) 3D FIB/SEM reconstruction shows the embedding of the cells in the collagen matrix (cyan). Discrete cells are represented with different colors. b) TEM image of a 70 nm section showing the random distribution of collagen fibrils. Collagen type I was identified by immunolabelling. c–e) Fluorescent immunohistochemistry identifying key non‐collagenous proteins in the collagenous matrix: c) Co‐localization of osteocalcin (green) and collagen (red). d) Osteopontin (green) distribution in the collagen matrix (red). * Indicates the silk fibroin scaffold. e) Co‐localization of DMP1 (green) with the collagen structure (see Figure S5, Supporting Information, for collagen image). f–g) Raman microspectrometry of mineralized matrices. f) Localized Raman spectra of mineralized collagen of developing zebrafish bone (red), the 3D osteogenic co‐culture (blue), and human bone of a 10 year old female (grey g) Raman derived mineral/matrix ratios of 4 mineralized tissues of zebrafish (N = 6, red), Osteogenic 3D culture (N = 7, green), 10 year old human female (N = 1, grey), and 48+ years old human male (N = 7, black, taken from ref. [36]). Bars indicate sample standard deviations. h) Heat map presentation of a 3D FIB/SEM cross section showing disorganized collagen fibrils with different degrees of mineralization (Figure S10, Supporting Information). Arrowheads indicate non‐mineralized collagen fibrils (light blue), arrow indicates mineralized collagen fibril (orange). i) TEM image showing individual mineralized collagen fibrils. Researchers grow most lifelike bone yet from woven cells](https://scx1.b-cdn.net/csz/news/800a/2021/4-researchersg.jpg)