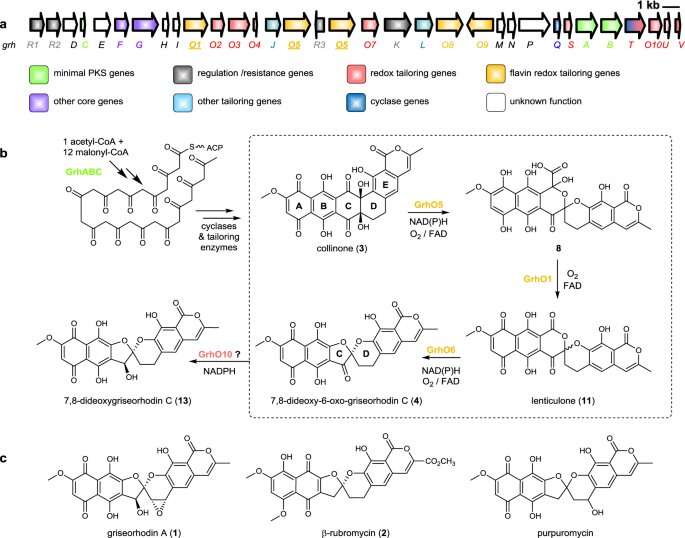
Overview of the proposed biosynthesis of bacterial rubromycin-type polyketides and final pathway products. Credit: Nature Communications (2021). DOI: 10.1038/s41467-021-21432-9
Plants, fungi and bacteria produce natural products that function as defenses that are deployed against predators and competitors. In medicine, these compounds have such applications as antibiotics, cancer drugs and cholesterol reducers. The team working with associate professor Dr. Robin Teufel and Dr. Britta Frensch of the Institute of Biology II of the Faculty of Biology of the University of Freiburg and researchers from the ETH Zürich in Switzerland were able to shed light on the key role of three enzymes that are involved in synthesizing a class of natural products. The researchers have published their findings in Nature Communications.
Actinobacteria produce many natural products, such as those that are known as aromatic polyketides. The Freiburg researchers examined how actinobacteria—aided by enzymes—were able to synthesize such bioactive substances from simple, molecular components. Teufel and his team were able to illuminate the key roles played by three enzymes in the biosynthesis of rubromycins, which belong to the most structurally complex aromatic polyketides.
The researchers discovered that the enzymes drastically restructure a chemical precursor molecule. Through this process they create the carbon backbone of the rubromycins, which is key to the diverse, pharmacological effects of these compounds. Using chemical and biochemical methods, the researchers succeeded in examining the functions of the enzymes more closely and identifying several previously unknown intermediates in the biosynthesis of the rubromycins.
Teufel explains, "We've made important findings about the ways such enzymes control the formation of complex natural products in microorganisms. These findings could play a central role in applying bioengineering to make new types of bioactive rubromycin-polyketides."
More information: Britta Frensch et al, Enzymatic spiroketal formation via oxidative rearrangement of pentangular polyketides, Nature Communications (2021). DOI: 10.1038/s41467-021-21432-9
Journal information: Nature Communications
Provided by University of Freiburg