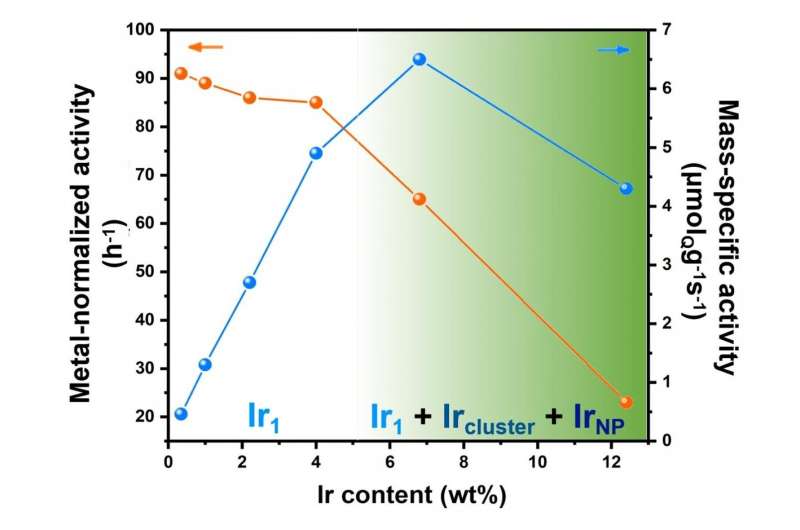
Metal-normalized activity and mass-specific activity of Ir/MoC catalysts with different Ir content. Ir1 stands for atomically dispersed Ir. The shade highlights that when Ir loading is lower than 4%, the dominant species are Ir1, while it gradually changes the mixture of Ir1, Ir clusters and Ir nanoparticles at higher Ir loading. Credit: Science China Press
Atomically dispersed catalysts have received extensive research attention, because they exhibit excellent activity and unique selectivity for many important catalytic reactions. The atomically dispersed nature of these metal catalysts confers their unique electronic structures as well as designated coordination-unsaturated environments for the optimized adsorption/activation of the reactants. One grand challenge faced by these atomically dispersed catalysts is that the supported isolated metalatoms are usually thermally unstable and tend to aggregate into large clusters/particles at evaluated reaction temperatures. As a result, most reported atomically dispersed catalysts have an extremely low metal loading below 1.5 wt%. Because of the extremely low metal loading, many atomically dispersed catalysts suffer from low mass-specific activity, which is often considered more crucial, especially in industrial applications. Therefore, developing new strategies for constructing atomically dispersed catalysts with high metal loading, high thermal stability, and high catalytic performance is of great importance.
In order to achieve high metal loading and high thermal stability, the support material should have a high specific surface area with abundant surface sites that could provide strong anchoring to the supported metal species. Meanwhile, for optimizing the catalytic performance, the support material should also be carefully chosen to tune the electronic properties of the supported species, and to participate in catalyzing the reaction. In a new article published in the Beijing-based National Science Review, scientists at the College of Chemistry and Molecular Engineering of Peking University in Beijing, China, and at University of Chinese Academy of Sciences in Beijing, China, and at University of Science and Technology of China in Hefei, China report a facile synthesis of a thermally stable atomically dispersed Ir/MoC catalyst with metal loading as high as 4 wt%, an unusually high value for carbide supported metal catalysts. The strong interaction between Ir and the MoC substrate enables high dispersion of Ir on the MoC surface, and modulates the electronic structure of the supported Ir species. Using quinoline hydrogenation as a model reaction, Ir/MoC catalyst exhibits remarkable reactivity, selectivity, and stability. The presence of high-density isolated Ir atoms is the key to achieve high metal-normalized activity and mass-specific activity, whereas MoC substrate contributes to block the unselective hydrogenation of benzene ring in quinoline at harsh reaction conditions. Based on theoretical calculations, the authors show that water-promoted quinoline hydrogenation mechanism is preferred over the Ir/MoC, which contributes to high selectivity towards 1,2,3,4-tetrahydroquinoline.
Noteworthy is that the authors pointed out the importance of metal loading for atomically dispersed catalyst based on their reaction data as "We can draw the conclusion that the Ir1 species on α-MoC surface are more reactive than Ir clusters or Ir NPs in this reaction, giving the highest metal-normalized activity on 0.5-4% Ir/α-MoC catalysts. We need to point out that very low metal loading of a supported metal catalyst can result in an extremely low mass-specific activity, which is a drawback in practical applications. In our view, high-loading atomically dispersed catalyst (e.g. 4% Ir/α-MoC) and catalyst with the highest density of isolated metal atom (e.g. 7% Ir/α-MoC) are significant for both academia and chemical industry."
More information: Siwei Li et al, Atomically dispersed Ir/α-MoC catalyst with high metal loading and thermal stability for water-promoted hydrogenation reaction, National Science Review (2021). DOI: 10.1093/nsr/nwab026
Provided by Science China Press