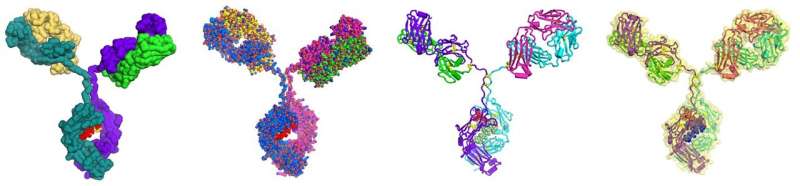
The NIST Monoclonal Antibody (NISTmAb). Credit: National Institute of Standards and Technology
A new method for monitoring biopharmaceutical product quality was recently put to the test by The National Institute of Standards and Technology (NIST) and 28 laboratories representing the biopharmaceutical industry, instrument and software vendors, and the federal government. The results of this interlaboratory study were recently published in the Journal of the American Society for Mass Spectrometry.
The new multi-attribute method, or MAM, is an emerging, mass spectrometry-based technique for monitoring product quality and detecting multiple types of potential impurities in biopharmaceutical products with a single-step test.
Participants evaluated MAM using the NIST Monoclonal Antibody (NISTmAb) Reference Material 8671 as a model test sample. They were given several altered NISTmAb samples and asked to report any differences found between those and the unaltered reference material.
The study showed that participating labs were able to successfully identify several types of alterations in the samples using MAM, including impurities and chemical transformations that have to be tightly controlled when producing biopharmaceutical products.
The study also identified the current capabilities and challenges associated with MAM and outlines best practices that can improve reliability.
"The ultimate goal is to speed the time-to-market for life-saving medicines through more efficient quality control methods," said NIST research chemist Trina Mouchahoir. "And this study provides a roadmap for getting there."
More information: Trina Mouchahoir et al. New Peak Detection Performance Metrics from the MAM Consortium Interlaboratory Study, Journal of the American Society for Mass Spectrometry (2021). DOI: 10.1021/jasms.0c00415
Provided by National Institute of Standards and Technology