February 9, 2021 feature
How virally derived transposons are domesticated to evolve new forms of life
About half of our genome is made up of transposable elements (TEs), also known as transposons. These 'jumping genes' are short stretches of DNA that have the unique ability to duplicate themselves and change their position within our code. While these philanderings play an essential role in the evolution of the species, if unchecked, transposons can wreak havoc on the genome.
Although the transcription and proliferation of TEs is usually constrained by DNA methylation or other repressive chromatin amendments, TEs sometimes escape these countermeasures. For example, at certain periods of germ cell gametogenesis and early embryonic development, many epigenetic controls are wiped clean during scheduled system-wide reboots. Fortunately, cells have a backup mechanism known as the PIWI/piRNA pathway which can repress TEs. A recent paper in Nature Reviews Molecular Cell Biology investigates the many ways in which piRNAs can silence TEs, and defines new mechanisms by which they might also control gene expression.
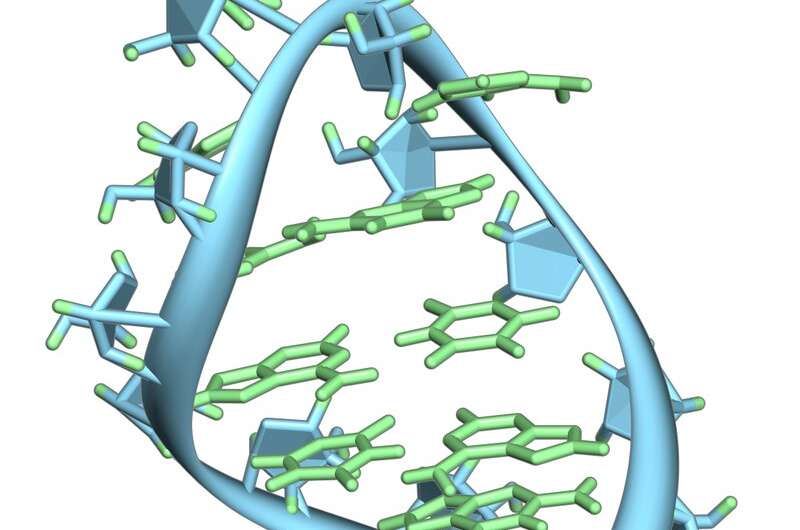
In the PIWI/piRNA pathway, RNA molecules about 25-32 nucleotides long associate with the Argonaute proteins from the PIWI clade to form piRISC complexes. These complexes target TEs post-transcriptionally, and also through the induction of epigenetic changes at the loci from which they are expressed. Piwi is an abbreviation of "P-element Induced WImpy testis," with the "P" meaning paternal. The aforementioned affliction was initially observed in drosophila. piRNA simply means PIWI interacting RNA. Transposon-silencing piRNA sequences tend to drift as older transposons decay, and new transposon invasions emerge that in turn require novel piRNAs to protect the germline genome. Positive selection therefore favors only piRNAs that target the youngest and most active transposons, and as a result, piRNA sequences rapidly diverge and become species-specific rather than ancestral. A convenient analogy for piRNAs is that they act as a kind of RNA-mediated adaptive immunity against runaway transposon expansions and invasions.
Two main classes of TEs can be defined. Class I TEs are called retrotransposons, which generally function via reverse transcription of the DNA into an RNA intermediate, hinting at their retroviral ancestry. Retrotransposons are commonly grouped into three main orders of decreasing size and complexity; the retrotransposons with long terminal repeats (LTRs), the LINES (which have reverse transcriptase but not LTRs), and the SINEs (which have neither). Class II TEs are the DNA transposons, which typically encode several sequence curiosities including a special transposase used for insertion and excision. SINEs (Short Interspersed Nucleotide Repeat Elements), like the Alu repeats that are abundant in primate genomes, are generally around 300 nucleotides long and are transcribed by RNA polymerase III. The Alu element was originally characterized by the action of the Arthrobacter luteus (Alu) restriction endonuclease.
Lest we give the impression that the transposon hazard is something we should be better off without, consider that transposons are solely responsible for most, or at least many, of the higher evolutionary refinements we enjoy today. Everything from live birth to expansion of the neocortex appears to have been driven by genome-wide insertion of TEs into the promoter regions of key regulatory genes. No other evolutionary process capable of radically altering the expression of so many genes in such a short time has been identified, let alone imagined.
The exaptation of seemingly randomly inserted endogenous retroviral (ERV) sequences into discrete, highly organized developmental functions has occurred with uncanny parallelization across different species. For example, mouse, human and rabbit have all independently co-opted envelope protein genes (Env) from different ERVs to act as syncytins in the creation of an invasive, fusogenic placenta. Independent capture of syncytins has occurred at least six times during mammalian evolution, and even marsupials, which have a relatively transient fused placenta that is in contact with the maternal endometrium for a short period of time. Syncytian-1 and syncytian-2 appear to have entered the primate genome approximately 25 million and 40 million years ago respectively.
The classic example of full integration of genome-wide transposable elements in the coordinating of body-wide systems was the generation of an alternative promoter directly controlling extra-pituitary prolactin expression during the evolutionary transition from oviparity to viviparity (egg laying to live live birth). Again, these events are highly parallelized across species, with the primate renditions involving the insertion of two separate TEs near the prolactin promoter region. The first (MER20 "MEdian Repeat 20'), belongs to a class of DNA transposons collectively known as MER1, and has been traced back to around 70–80 million years, before the mammalian radiation. The second, named MER39, is a retrotransposon derived from the ERV1 class. It is present in chimps and macaques but absent from dogs, mice, and rats, and therefore likely inserted at least 25–30 million years ago, before the divergence of Old World monkeys from higher apes.
The magic sauce hidden in these transposable elements is that they happen to be good at creating transcription factor binding sites. These sites have been shown to mediate prolactin transcription first in lymphocytes, and later in uterine decidualized endometrial cells. Freed of the constraints of pituitary prolactin expression, decidual prolactin (dPRL) could divergently evolve to control many pregnancy-specific functions like immune tolerance of the embryo, and ultimately, lactation. In addition to a direct function in promoter regions, TEs end up acting in enhancer, insulator and repressor roles. In embryonic stem cells, the MER20 transposon is an enhancer for the prolactin gene, which is just one of many progesterone/cAMP-responsive genes found in these cells.
While suppression of immune function is critical for tolerating an invasive placenta, other extant genes continue to be tamed in pregnancy. For example, many ion transporters and channels once important in eggshell mineralization, like ATP2B2, SLC12A5, SLC12A8, SLC26A9, and TRPV5, have entirely lost endometrial expression in mammals. Of the approximately 1,500 genes found to be recruited into endometrial expression in placental mammals, about 13% of them have a Eutherian-specific MER20 transposon within 200 kb. The full extent of small RNAs, like the PIWI-piRNA class, in the regulation of these kinds of transposons remains to be seen.
In the case of the PRL gene, an alternative initial exon does not contain coding sequence, and therefore transcription from the alternative promoter does not lead to the generation of protein isoforms. This extra exon could have contributed to the expansion of the transcriptional and translational profile of the PRL gene. The use of alternative promoters is hardly an uncommon phenomenon. According to some estimates, almost half of the human genome is predicted to be transcribed via multiple promoters. While in many instances, alternative promoters confer tissue-specific expression, in other cases, they confer organelle-specific expression. Although nuclear localization sequences are often found at the tail end of a gene, the mitochondrial localization sequences (MLS) typically result from alternative splicing of the initial exon of mitochondrial localized isoforms.
This all suggests the intriguing possibility that the origins and proliferation of MLS sequences, which until now has occurred by some completely unimagined evolutionary mechanism, might derive from the actions of transposable elements similar to those witnessed in the evolution of progesterone/cAMP responsive genes, like prolactin.
More information: Pei-Hsuan Wu et al. Defining the functions of PIWI-interacting RNAs, Nature Reviews Molecular Cell Biology (2021). DOI: 10.1038/s41580-021-00336-y
© 2021 Science X Network