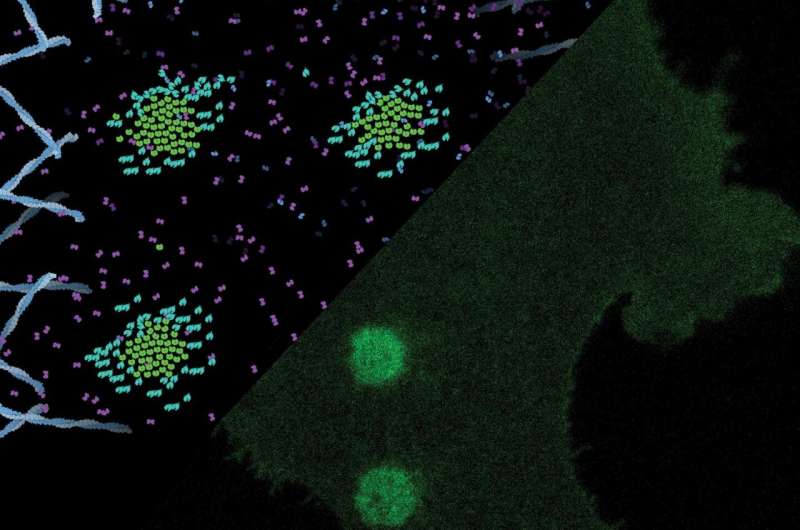
Laser spots activate very small synthetic lock-and-key pairs in a matrix to create receptor clusters in the cell membrane. This ligand-independent activation triggers calcium signaling and increased cell motility. (Graphic copyright: M. Florencia Sánchez & Robert Tampé, Goethe University Frankfurt.) Credit: M. Florencia Sánchez & Robert Tampé, Goethe University Frankfurt
Body cells communicate with each other, receive signals from the outside world and react to them. A central role in this communication network is attributed to receiver proteins, called receptors, that are anchored at the cell membrane. There, they receive and transmit signals to the inside of the cell, where a cell reaction is triggered.
In humans, G-protein-coupled receptors (GPC receptors) represent the largest group of these receptor molecules, with around 700 different types. New research by Frankfurt and Leipzig scientists focused on a GPC receptor that serves as a receptor for the neuropeptide Y in cells and is accordingly called the Y2 receptor. Neuropeptide Y is a messenger substance that primarily mediates signals between nerve cells, which is why Y2 receptors are mainly present in nerve cells and among other activities trigger the formation of new cell connections.
In the laboratory, the researchers engineered cells, which had approx. 300,000 Y2 receptors on their surface and were grown on specifically developed, light-sensitive matrices. Each of the Y2 receptors was provided with a small molecular "label." Once the scientists created a spot of light with a fine laser beam on the cell surface, the Y2 receptors under this spot were trapped via the molecular label to the exposed matrix in such a way that the they moved closely together to form an assembly known as a cluster. The whole reaction could be immediately observed at the defined spot and within a few seconds.
Professor Robert Tampé from the Institute of Biochemistry at Goethe University Frankfurt explains: "The serendipity of this experiment is that the clustering of receptors triggers a signal that is similar to that of neuropeptide Y. Solely by the clustering, we were able to trigger cell movement as a reaction of the cell. The laser spots even allowed us to control the direction of the cell movement."
As the light-sensitive lock-and-key pairs are very small compared to the receptors, the organization of the receptors in the cell membrane can be controlled with high precision using the laser spot. "This non-invasive method is thus particularly well suited to study the effects of receptor clustering in living cells," Tampé continues. "Our method can be used to investigate exciting scientific questions, such as how receptors are organized in networks and how new circuits are formed in the brain."
Upon irradiation with laser light (white rings), receptors cluster in the cell (light green circles). Thereupon, the cell moves into the direction of the receptor clusters. Credit: M. Florencia Sánchez & Robert Tampé, Goethe University Frankfurt. Reprinted with permission from M. F. Sánchez et al., Science 10.1126/science.abb7657(2021)
More information: M. Florencia Sánchez et al, Photo-induced receptor confinement drives ligand-independent GPCR signaling, Science (2021). DOI: 10.1126/science.abb7657
Journal information: Science
Provided by Goethe University Frankfurt am Main